Abstract
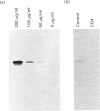
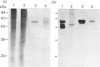
A monoclonal antibody, CG4, was raised to a novel 60 kDa metalloproteinase purified from a bovine brain myelin glycoprotein fraction. Glycoproteins extracted from both myelin and nine different bovine tissues showed the 60 kDa CG4-immunoreactive band by immunoblotting in amounts that broadly paralleled enzymic activity of this metalloproteinase and varied relatively little among the tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beynon R. J., Shannon J. D., Bond J. S. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J. 1981 Dec 1;199(3):591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chantry A., Earl C., Groome N., Glynn P. Metalloendoprotease cleavage of 18.2- and 14.1-kilodalton basic proteins dissociating from rodent myelin membranes generates 10.0- and 5.9-kilodalton C-terminal fragments. J Neurochem. 1988 Mar;50(3):688–694. doi: 10.1111/j.1471-4159.1988.tb02968.x. [DOI] [PubMed] [Google Scholar]
- Chantry A., Gregson N. A., Glynn P. A novel metalloproteinase associated with brain myelin membranes. Isolation and characterization. J Biol Chem. 1989 Dec 25;264(36):21603–21607. [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Bowes M. A., Buck P., Kenny A. J. An immunoradiometric assay for endopeptidase-24.11 shows it to be a widely distributed enzyme in pig tissues. Biochem J. 1985 May 15;228(1):119–126. doi: 10.1042/bj2280119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn P., Chantry A., Groome N., Cuzner M. L. Basic protein dissociating from myelin membranes at physiological ionic strength and pH is cleaved into three major fragments. J Neurochem. 1987 Mar;48(3):752–759. doi: 10.1111/j.1471-4159.1987.tb05581.x. [DOI] [PubMed] [Google Scholar]
- Groome N., Chantry A., Earl C., Newcombe J., Keen J., Findlay J., Glynn P. A new epitope on human myelin basic protein arising from cleavage by a metalloendoprotease associated with brain myelin membranes. J Neuroimmunol. 1988 Aug;19(1-2):77–88. doi: 10.1016/0165-5728(88)90037-9. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Bowes M. A., Gee N. S., Matsas R. Endopeptidase-24.11: a cell-surface enzyme for metabolizing regulatory peptides. Biochem Soc Trans. 1985 Apr;13(2):293–295. doi: 10.1042/bst0130293. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Ingram J. Proteins of the kidney microvillar membrane. Purification and properties of the phosphoramidon-insensitive endopeptidase ('endopeptidase-2') from rat kidney. Biochem J. 1987 Jul 15;245(2):515–524. doi: 10.1042/bj2450515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Newcombe J., Woodroofe M. N., Cuzner M. L. Distribution of glial fibrillary acidic protein in gliosed human white matter. J Neurochem. 1986 Dec;47(6):1713–1719. doi: 10.1111/j.1471-4159.1986.tb13079.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]