Abstract
Introduction
Oral antifungals are the treatment choice for onychomycosis, and topical therapies are favored in cases of limited nail involvement. Recently, carbon dioxide (CO2) laser treatment has emerged as an option to enhance the effectiveness of topical therapies.
Objectives
Our objective was to compare the efficacy of fractional ablative and fully ablative CO2 laser treatments for distolateral subungual onychomycosis affecting a single toenail and caused by dermatophytes.
Methods
The records of 10 patients treated with a single fully ablative CO2 session were matched with those of 10 patients who underwent a single CO2 fractional treatment. All had previously failed topical antifungal lacquers and were discharged with the prescription of topical ciclopirox nail lacquer (8%) for 3 months.
Results
The clinical response rates were 80% for the fully ablative group and 60% for the fractional group. Additionally, the mean reduction in Onychomycosis Severity Index from baseline to 8.6±1.6 weeks after treatment completion was 6.9±5.4 in the fully ablative group and 3.6±6.6 in the fractional group. The relapse rate among responders was 12.5% in the fully ablative and 33.3% in the fractional group after a mean follow-up time of 29.4±2.3 weeks.
Conclusions
Fractional and fully ablative CO2 laser in combination with ciclopirox lacquer could increase the response rate in onychomycosis resistant to topical antifungals when systemic therapy is contraindicated or not yet pursued. Fully ablative mode therapy is significantly more effective than fractional (P < 0.05). Further studies are needed to identify prognostic response factors and assess the long-term effectiveness of CO2 laser treatment.
Keywords: onychomycosis, laser, nail, treatment, physical therapy
Introduction
Onychomycosis is the most prevalent nail infection, affecting nearly 5.5% of the global population [1]. Causative organisms include dermatophytes, non-dermatophyte molds, and yeasts. Dermatophyte infections constitute the majority, primarily attributable to Trichophyton rubrum and T. mentagrophytes var. interdigitale [2]. According to the modality of nail invasion, onychomycosis can be categorized into 4 primary types, namely: distolateral subungual onychomycosis, proximal subungual onychomycosis, superficial onychomycosis, and total dystrophic onychomycosis. A fifth class, endonyx subungual onychomycosis, is relatively rare [3]. Risk factors that predispose individuals to this infection include advanced age, repeated nail trauma, psoriasis, diabetes, peripheral circulatory disorders, and immunosuppression. Additionally, the simultaneous presence of plantar or interdigital tinea pedis is considered a risk factor that can co-occur with onychomycosis [4].
Distolateral subungual onychomycosis (DLSO), the most common type, usually affects the toenails, particularly the big toes. Common clinical presentations include nail detachment (onycholysis), changes in nail color (yellow, white, or brown), and subungual hyperkeratosis [5]. Furthermore, affected nails can cause pain or paresthesia, significantly impacting the patient quality of life [6]. Onychoscopy typically reveals longitudinal white-yellow striae and an irregular proximal margin of onycholytic areas with several spikes [7].
The diagnosis of onychomycosis is established through various techniques, including direct microscopy of subungual scales, fungal culture, histopathology, PCR, and flow cytometry [8]. It is highly recommended to isolate the fungal pathogens to optimize therapy [9]. After diagnosis, selecting the most suitable treatment is crucial. The choice of treatment initially depends on the number of affected nails. Systemic therapy is preferred for cases involving more than three nails [2].
For DLSO due to dermatophytes, oral terbinafine at 250 mg/day (or in pulse administration at the dose of 500 mg/day for 1 week per month) for 12 weeks is considered the gold standard [10,11]. Itraconazole in a pulse regime (400 mg/day for 1 week per month) for 12 weeks is prescribed for patients who exhibit intolerance or inadequate response to oral terbinafine or when onychomycosis is caused by non-dermatophytic molds or yeasts [12,13].
Topical treatment is indicated for DLSO affecting three or fewer nails and up to 40% nail involvement [14]. Several topical antifungals in different vehicles are available, including ciclopirox 8%, amorolfine 5%, efinaconazole 10%, tavaborole 5%, and terbinafine 10% [15]. Combination therapy, involving both topical and oral treatments, may yield better results in challenging cases [14,16]. An emerging concern is terbinafine-resistant fungal infections, associated with mutations in the squalene oxidase gene, with the highest prevalence reported in Western India at 77% [17]. This resistance affects both cutaneous tinea and tinea unguium in various European countries [18,19]. The occurrence of drug resistance in dermatophyte infections underscores the relevance of combining systemic or topical drugs with physical treatments [20,21].
Several device-based therapies may be useful for these resistant cases, either directly targeting the fungus or acting as adjuvants to enhance the effectiveness of topical and oral medications. These devices encompass lasers, photodynamic therapy (PDT), iontophoresis, ultrasound, micro-drilling, plasma, and microwaves [22]. Laser devices, such as carbon dioxide (CO2) lasers, neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers, and diode lasers, emit specific wavelengths of light to penetrate the nail and target fungal cells [23]. PDT involves applying a photosensitizing agent followed by exposure to specific light wavelengths to produce reactive oxygen species that kill fungal cells [24]. Iontophoresis facilitates the movement of positively charged drugs to the negatively charged area beneath the nail plate [25]. Ultrasound creates small cavities and pits in the nail surface, enhancing the penetration of antifungal medications or agents and potentially improving treatment outcomes [26]. Micro-drilling involves creating microphores on the nail plate surface leading to the nail bed, forming reservoirs for topical drugs [13]. Plasma therapy uses growth factors and bioactive substances in the plasma to promote healthy nail growth and combat fungal infection [27]. Microwave therapy for onychomycosis utilizes microwave energy to heat the affected nail and surrounding tissues, targeting the fungal infection. The goal is to raise the temperature sufficiently to eliminate the fungus while minimizing damage to healthy tissues [28].
To assess the severity of DLSO, Carney et al validated the Onychomycosis Severity Index (OSI) [29]. This straightforward scoring system evaluates subungual hyperkeratosis, proximity of the infection to the matrix, percentage of nail plate involvement, and the presence of dermatophytomas. Since treatment efficacy is established by clinical and mycological cure, the OSI could serve as an objective and standardized outcome measure.
Clinical cure indicates the growth of a normal nail with no residual signs of nail plate changes related to onychomycosis, while mycological cure is achieved by negative mycology (ie negative potassium hydroxide (KOH) microscopic examination and negative fungal cultures [2].
Objectives
The goal of our retrospective study was to compare the efficacy of fractional and full-ablative CO2 laser as adjunctive treatment to topical ciclopirox 8% nail lacquer for DLSO caused by dermatophytes.
Methods
We retrieved and matched the records of 20 patients treated with ciclopirox 8% nail lacquer (®Niogermox) for 12 weeks, preceded respectively by a single session of fractional (10 patients) and full ablative (10 patients) CO2 SmartXide Punto laser (DEKA- M.E.L.A-), performed in a single Hospital Institution between January 2022 and December 2022.
Inclusion criteria were as follows: onychomycosis affecting a single big toe, with involvement ranging from 25% to 75% of the nail plate, a lack of response to topical nail lacquer treatment continued for a minimum of 3 months, and a positive mycological test confirming the presence of the fungus before initiating laser treatment. Additionally, all patients either declined systemic treatments or had contraindications for such treatments. Prior to participating in the study, all patients provided written informed consent. All examinations were conducted in accordance with the Helsinki principles of medical ethics.
The treatment protocols encompassed fractional CO2 laser sessions, utilizing the superpulse mode with an output power of 15 W, density at 17.1%, pulse width of 1200 μs, dot spacing set at 400 μm, and a stack of 3 (resulting in an irradiation output power of 15.40 J/cm2) for 10 patients. The other 10 patients underwent full-ablative CO2 laser treatment, employing the superpulse mode with an output power ranging from 6 to 8 W, a single pass, a frequency of 10 Hz, and pulse energy set at 600 mJ. The endpoint of the full-ablative treatment was the ablation of the superficial (dorsal) layer of the nail plate while preserving the deeper layer (ventral plate). To ensure patient comfort, a distal digital block was administered with a 2% lidocaine solution 15 minutes before the procedure. Following the laser session, ciclopirox 8% nail lacquer was applied with occlusion for 24 hours, followed by daily application without occlusion for the subsequent 12 weeks. The follow-up visits were categorized into short-term assessments to evaluate laser efficacy and long-term assessments to monitor potential relapse. We employed the OSI score system to determine the severity of onychomycosis.
For each patient, we collected the following data: gender, age, comorbidities, disease duration, causative fungal species, prior therapies, clinical and mycological cure rates, response rates, and relapse occurrences within the long-term (6–9 months) follow-up after completing the topical treatment. Clinical cure was defined as achieving at least an 80% reduction in the basal OSI score, partial cure was set by an OSI decrease ranging from 50–80% of the initial value, while mycological cure required negative culture and negative direct microscopy results.
Statistical analyses were conducted using IBM SPSS Statistics V.23. Frequencies were used to report qualitative data while mean and standard deviation summarized quantitative data. The student t test or Mann-Whitney test for independent means was used, as appropriate, to compare scale variables according to a parametric or nonparametric distribution. For all analyses, a P-value less than or equal to 0.05 was considered significant.
Results
As reported in Table 1 the study included 10 males and 10 females, with an average age of 61.9 years, ranging from 32 to 82 years. The mean duration of DLSO was 7.1 months, ranging from 2 to 13 months. T. rubrum was identified in 16 patients (80%), whereas T. interdigitale was found in the remaining 4 cases (20%). The most common comorbidities were type 2 diabetes mellitus (T2DM) 25%, hypertension (HTN) 25%, chronic venous insufficiency (10%), lateral deviation of the hallux (10%), and hyperthyroidism (10%).
Table 1.
Demographics, Comorbidity Factors, Treatment Modalities, and Treatment Outcomes of Patients in This Study
| Patient # | Gender | Age, years | Comorbidities | Disease Duration, months | Fungi Species | Mode of CO2 Laser | OSI at T0 | OSI at T1 | Mycological Cure at T1 | Clinical Cure at T1 | Relapse at T2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 55 | Chronic kidney disease | 13 | T. rubrum | CW | 6 | 1 | negative | CR | none |
| 2 | M | 43 | Psoriasis, chronic myeloid leukemia | 11 | T interdigitale | CW | 10 | 1 | negative | CR | none |
| 3 | M | 78 | Chronic venous insufficency | 8 | T. rubrum | CW | 8 | 1 | negative | CR | none |
| 4 | M | 64 | T2DM, HTN | 10 | T. rubrum | CW | 12 | 1 | negative | CR | none |
| 5 | M | 65 | HTN | 5 | T. rubrum | CW | 9 | 2 | negative | PR | none |
| 6 | F | 77 | Hypothyroidism, vitiligo | 7 | T. rubrum | CW | 6 | 0 | negative | CR | none |
| 7 | F | 82 | Metabolic syndrome | 9 | T. rubrum | CW | 16 | 4 | negative | PR | present |
| 8 | F | 32 | HTN, Hypercholesterolemia | 10 | T. rubrum | CW | 16 | 2 | negative | CR | none |
| 9 | F | 34 | Lateral deviation of the hallux | 9 | T. rubrum | CW | 9 | 6 | positive | NR | / |
| 10 | F | 52 | Hypothyroidism | 2 | T. rubrum | CW | 3 | 8 | positive | NR | / |
| 11 | M | 58 | - | 3 | T. rubrum | FSP | 6 | 1 | negative | CR | none |
| 12 | M | 62 | T2DM, HTN | 7 | T. rubrum | FSP | 16 | 12 | positive | NR | / |
| 13 | M | 49 | Psychosis | 4 | T. rubrum | FSP | 2 | 0 | negative | CR | none |
| 14 | M | 80 | T2DM | 11 | T interdigitale | FSP | 9 | 16 | positive | NR | / |
| 15 | M | 71 | T2DM | 7 | T. rubrum | FSP | 12 | 15 | positive | NR | / |
| 16 | F | 69 | HTN | 6 | T. rubrum | FSP | 16 | 2 | negative | CR | present |
| 17 | F | 68 | T2DM | 5 | T. rubrum | FSP | 8 | 4 | negative | PR | none |
| 18 | F | 79 | Chronic venous insufficency | 8 | T interdigitale | FSP | 20 | 6 | negative | PR | present |
| 19 | F | 66 | Lateral deviation of the hallux | 4 | T. interdigitale | FSP | 8 | 8 | positive | NR | / |
| 20 | F | 54 | - | 3 | T. rubrum | FSP | 3 | 0 | negative | CR | none |
CR = complete response; CW = continuous wave; F = female; FSP = fractional super-pulse; HTN = hypertension; M = male; PR = partial response; T0 = baseline parameters; T1 = short-term follow up; T2 = long-term outcome; T2DM = type 2 diabetes mellitus.
At baseline, the median OSI was 9.9±4.2 in patients who underwent treatment with a fully ablative CO2 laser, and 10±5.9 in those treated with fractional mode. At the first follow-up visit, which occurred 8.6±1.6 weeks after the 12 week-completion of the ciclopirox 8% lacquer, the mean difference of the OSI before and after treatment was 6.9±5.4 in the fully ablative group and 3.6 ±6.6 in the fractional group (Table 1). The difference of OSI score among the two groups (2.78±2.59 vs. 6.4 ± 6.11) at the end of the study was statistically significant (P < 0.05). The overall clinical response matched with the mycological cure and was computed as 80% versus 60% in the fully ablative compared to the fractional group, with a rate of 60% and 30% of complete responders among the 2 groups (Table 2). The relapse rate among the responders (14/20) was 12.5% in the fully ablative and 33.3% in the fractional group after a mean follow-up time of 29.4±2.3 weeks. No adverse events were reported during the study.
Table 2.
Clinical and Mycological Cure Rate at T1 and Relapse Rate at T2 in Both Fully Ablative (CW) and Fractional Groups (FSP).
| Complete Clinical Response at T1 | Partial Clinical Response at T1 | No Clinical Response at T1 | Mycological Cure at T1 | Relapse Rate at T2 | |
|---|---|---|---|---|---|
| CW | 60% | 20% | 20% | 80% | 12.5% |
| FSP | 30% | 30% | 40% | 60% | 33.3% |
CW = continuous wave; FSP = fractional super-pulse
Table 2 shows clinical and mycological rate of both fully ablative and fractional groups at short-term follow-up and relapse rate at 6-month follow up. In both groups, non-responders were offered systemic therapies. In cases where first-line and second-line systemic therapies were contraindicated, patients had the option to receive PDT, but some patients discontinued any treatment. Consequently, long-term follow-up data for this group were not included in the analysis.
Conclusions
Laser therapy has emerged as a viable treatment option for onychomycosis. In January 2012, the FDA approved 4 laser systems for “temporarily increasing clear nail in onychomycosis”: Nd:YAG (both long-pulsed and short-pulsed quality-switched), carbon dioxide laser, and diode lasers at 870 and 930 nm [2]. Laser treatment involves the use of specific wavelengths to target and destroy fungal cells through photo-thermolysis or thermal injury, while minimizing damage to surrounding healthy tissues, similar but more specific as compared to microwave-based devices [30]. The success of laser treatment for onychomycosis depends on several factors such as the severity of the infection, the type of fungus involved, the number of treatment sessions, and the expertise of the operator [23,31].
CO2 lasers find widespread use in dermatology for various procedures, including skin resurfacing and removal of benign skin lesions. CO2 laser treatment has been explored as a potential therapy for onychomycosis in both full-ablative and fractional modes. In the context of onychomycosis, full-ablative CO2 laser treatment works with a similar principle as other laser therapies, where the laser energy is absorbed by fungal cells, leading to their destruction [32]. In contrast, the fractional mode creates micro-holes on the nail plate surface, serving as pathways for topical antifungal delivery for at least six months following the procedure. This approach aims to enhance treatment efficacy by improving the absorption of medication through the laser-created micro-holes [33].
Several studies have shown promising results with CO2 laser treatment for onychomycosis. For instance, Weiwei et al reported a higher cure rate with full-ablative CO2 laser treatment (95%) compared to fractional mode (45%). The efficacy of CO2 laser lies in its ability to sterilize an area by raising local temperatures, effectively decomposing infected tissue [23]. Furthermore, combination therapy involving CO2 laser and topical treatments has demonstrated positive outcomes. Shi et al. used fractional CO2 laser in combination with terbinafine hydrochloride 1% cream, achieving mycological clearance rates of 77.4% and 74.2% at one month and three months post-treatment (34). In another study, Lee et al combined fractional CO2 laser with various topical antifungal agents, resulting in clinical responses (CR and PR) of 15.6% and 25%, respectively. They recommended more than 10 treatment sessions and a 12-month evaluation period for assessing treatment effectiveness [35]. A meta-analysis by Han et al confirmed the higher clinical improvement and mycological clearance rates achieved with combined CO2 laser therapy and topical agents compared to topical agents alone [36]. A study by Ortner and coworkers demonstrated that fractional laser ablation significantly improved the permeation of a fluorescent dye within both healthy and fungal nail tissue, with the greatest enhancement observed in the uppermost nail layer [37].
In our study, patients treated with fully ablative CO2 laser, combined with topical antifungals achieved higher rates of clinical and mycological cure (Figures 1–3 and Table 2). Conversely, fractional laser treatment led to a noticeable and gradual reduction in onycholysis-related striae and spikes around the microthermal zones at onychoscopy (Figures 4 and 5), but lesser percentages of clinical and mycological cures were recorded, along with a 33.3% relapse rate during the long-term follow-up. A possible explanation for the lower response rate of the fractional group is the previous failure of topical drugs.
Figure 1.
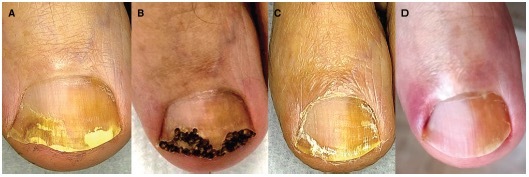
Patient #2, treated with fully ablative CO2 laser. (A) Clinical image at T0: OSI 10. (B) After fully ablative laser. (C) At T1: OSI 1. Mycological cure: negative, clinical cure: complete response (CR). (D) At T2: OSI 0. Mycological cure: negative, clinical cure: CR. OSI = Onychomycosis Severity Index.
Figure 2.
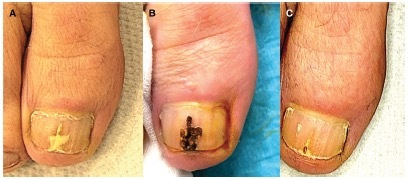
Patient #3, treated with fully ablative CO2 laser. (A) Clinical image at T0: OSI 8. (B) After fully ablative laser session. (C) At T1: OSI 1. Mycological cure: negative, clinical cure: complete response. OSI = Onychomycosis Severity Index.
Figure 3.
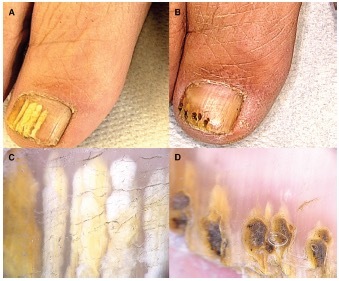
Patient #8, treated with fully ablative CO2 laser. (A,C) Clinical image and onychoscopy at T0: OSI 16. (B,D) At T1 OSI 2. Mycological cure: negative, clinical cure: complete response. OSI = Onychomycosis Severity Index.
Figure 4.
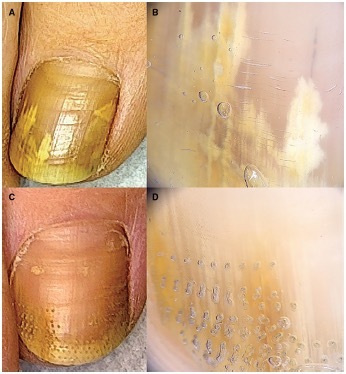
Patient #16, treated with fractional CO2 laser. (A,B) Clinical image and onychoscopy at T0: OSI 16. (C,D) At T1: OSI 2. Mycological cure: negative, clinical cure: complete response. OSI = Onychomycosis Severity Index.
Figure 5.
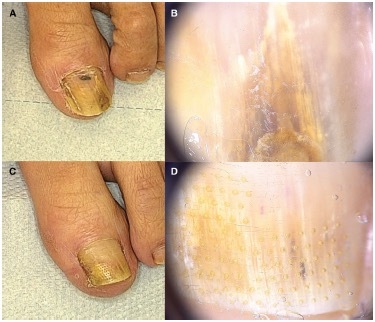
Patient #18, treated with fractional CO2 laser. (A,B) Clinical image and onychoscopy at T0: OSI 20. (C,D) At T1 OSI 6. Mycological cure: negative, clinical cure: partial response. OSI = Onychomycosis Severity Index.
Notably, the literature reports cure rates for key topical antifungal agents as follows: amorolfine at 30%, ciclopirox at 10%–25%, and tioconazole at 22% (38–42), which is far lower than the 70% and 40% rate of overall responders who did not relapse, which could be considered as cured.
Our patients received a single laser session preceding topical antifungal application. For non-responders or partial responders, additional laser sessions may be necessary to achieve remission. Also, clinical risk factors such as diabetes, chronic venous insufficiency and baseline disease severity could have impacted the response rate in our series. However, the limited sample size did not allow us to perform a regression analysis to correlate clinical response with patient characteristics.
The use of CO2 laser as an adjunctive therapy for onychomycosis has demonstrated promising outcomes by enhancing the penetration of topical agents. Fractional mode laser treatment may reduce visible signs of infection, while ablative laser procedures effectively vaporize and eliminate infected tissue, leading to improved sterilization and facilitating nail lacquer penetration. To prevent relapses, it could be advisable to extend the application of topical therapy over an extended duration or repeat laser sessions. When compared to conventional topical and oral therapies, laser treatment presents a more promising option for individuals with multiple comorbidities, elderly patients facing drug intolerance issues, as well as those with severe liver and kidney disease. However, additional research is warranted to identify prognostic response factors and assess the long-term effectiveness of CO2 laser treatment for onychomycosis.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Gupta AK, Versteeg SG, Shear NH. Onychomycosis in the 21st Century: An Update on Diagnosis, Epidemiology, and Treatment. J Cutan Med Surg. 2017;21(6):525–539. doi: 10.1177/1203475417716362. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Stec N, Summerbell RC, et al. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020;34(9):1972–1990. doi: 10.1111/jdv.16394. [DOI] [PubMed] [Google Scholar]
- 3.Westerberg DP, Voyack MJ. Onychomycosis: Current trends in diagnosis and treatment. Am Fam Physician. 2013;88(11):762–770. [PubMed] [Google Scholar]
- 4.Tosti A, Hay R, Arenas-Guzmán R. Patients at risk of onychomycosis--risk factor identification and active prevention. J Eur Acad Dermatol Venereol. 2005;19(Suppl 1):13–6. doi: 10.1111/j.1468-3083.2005.01282.x. [DOI] [PubMed] [Google Scholar]
- 5.Lipner SR, Scher RK. Onychomycosis: Clinical overview and diagnosis. J Am Acad Dermatol. 2019;80(4):835–851. doi: 10.1016/j.jaad.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 6.Lipner SR, Scher RK. Onychomycosis: current and investigational therapies. Cutis. 2014;94(6):E21–24. [PubMed] [Google Scholar]
- 7.Litaiem N, Mnif E, Zeglaoui F. Dermoscopy of Onychomycosis: A Systematic Review. Dermatol Pract Concept. 2023;13(1):e2023072. doi: 10.5826/dpc.1301a72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta AK, Hall DC, Cooper EA, Ghannoum MA. Diagnosing Onychomycosis: What’s New? J Fungi (Basel) 2022;8(5):464. doi: 10.3390/jof8050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta AK, Versteeg SG, Shear NH. Confirmatory Testing Prior to Initiating Onychomycosis Therapy Is Cost-Effective. J Cutan Med Surg. 2018;22(2):129–141. doi: 10.1177/1203475417733461. [DOI] [PubMed] [Google Scholar]
- 10.Takahata Y, Hiruma M, Shiraki Y, Tokuhisa Y, Sugita T, Muto M. Treatment of dermatophyte onychomycosis with three pulses of terbinafine (500 mg day for a week) Mycoses. 2009;52(1):72–76. doi: 10.1111/j.1439-0507.2008.01531.x. [DOI] [PubMed] [Google Scholar]
- 11.Lipner SR, Scher RK. Onychomycosis: Treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80(4):853–867. doi: 10.1016/j.jaad.2018.05.1260. [DOI] [PubMed] [Google Scholar]
- 12.Foley K, Gupta AK, Versteeg S, Mays R, Villanueva E, John D. Topical and device-based treatments for fungal infections of the toenails. Cochrane Database Syst Rev. 2020;1(1):CD012093. doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung AKC, Lam JM, Leong KF, et al. Onychomycosis: An Updated Review. Recent Pat Inflamm Allergy Drug Discov. 2020;14(1):32–45. doi: 10.2174/1872213X13666191026090713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta AK, Daigle D, Foley KA. Topical therapy for toenail onychomycosis: an evidence-based review. Am J Clin Dermatol. 2014;15(6):489–502. doi: 10.1007/s40257-014-0096-2. [DOI] [PubMed] [Google Scholar]
- 15.Blume-Peytavi U, Tosti A, Falqués M, et al. A multicentre, randomised, parallel-group, double-blind, vehicle-controlled and open-label, active-controlled study (versus amorolfine 5%), to evaluate the efficacy and safety of terbinafine 10% nail lacquer in the treatment of onychomycosis. Mycoses. 2022;65(4):392–401. doi: 10.1111/myc.13392. [DOI] [PubMed] [Google Scholar]
- 16.Maskan Bermudez N, Rodríguez-Tamez G, Perez S, Tosti A. Onychomycosis: Old and New. J Fungi (Basel) 2023;9(5):559. doi: 10.3390/jof9050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebert A, Monod M, Salamin K, et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses. 2020;63(7):717–728. doi: 10.1111/myc.13091. [DOI] [PubMed] [Google Scholar]
- 18.Shen JJ, Arendrup MC, Verma S, Saunte DML. The Emerging Terbinafine-Resistant Trichophyton Epidemic: What Is the Role of Antifungal Susceptibility Testing? Dermatology. 2022;238(1):60–79. doi: 10.1159/000515290. [DOI] [PubMed] [Google Scholar]
- 19.Saunte DML, Hare RK, Jørgensen KM, et al. Emerging Terbinafine Resistance in Trichophyton: Clinical Characteristics, Squalene Epoxidase Gene Mutations, and a Reliable EUCAST Method for Detection. Antimicrob Agents Chemother. 2019;63(10):e01126–19. doi: 10.1128/AAC.01126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bortoluzzi P, Prigitano A, Sechi A, et al. Report of terbinafine resistant Trichophyton spp. in Italy: Clinical presentations, molecular identification, antifungal susceptibility testing and mutations in the squalene epoxidase gene. Mycoses. 2023;66(8):680–687. doi: 10.1111/myc.13597. [DOI] [PubMed] [Google Scholar]
- 21.Gupta AK, Talukder M, Carviel JL, Cooper EA, Piguet V. Combatting antifungal resistance: Paradigm shift in the diagnosis and management of onychomycosis and dermatomycosis. J Eur Acad Dermatol Venereol. 2023 doi: 10.1111/jdv.19217. [DOI] [PubMed] [Google Scholar]
- 22.Gupta AK, Hall DC, Simkovich AJ. How Effective Are Devices in the Management of Onychomycosis?: A Systematic Review. J Am Podiatr Med Assoc. 2023;113(1):21–240. doi: 10.7547/21-240. [DOI] [PubMed] [Google Scholar]
- 23.Ma W, Si C, Kasyanju Carrero LM, et al. Laser treatment for onychomycosis: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98(48):e17948. doi: 10.1097/MD.0000000000017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen JJ, Jemec GBE, Arendrup MC, Saunte DML. Photodynamic therapy treatment of superficial fungal infections: A systematic review. Photodiagnosis Photodyn Ther. 2020;31:101774. doi: 10.1016/j.pdpdt.2020.101774. [DOI] [PubMed] [Google Scholar]
- 25.Martins Andrade JF, da Cunha Miranda T, Cunha-Filho M, Taveira SF, Gelfuso GM, Gratieri T. Iontophoresis application for drug delivery in high resistivity membranes: nails and teeth. Drug Deliv Transl Res. 2023;13(5):1272–1287. doi: 10.1007/s13346-022-01244-0. [DOI] [PubMed] [Google Scholar]
- 26.Kline-Schoder A, Le Z, Zderic V. Ultrasound-Enhanced Ciclopirox Delivery for Treatment of Onychomycosis. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:5717–5720. doi: 10.1109/EMBC.2018.8513552. [DOI] [PubMed] [Google Scholar]
- 27.Lokajová E, Julák J, Khun J, et al. Inactivation of Dermatophytes Causing Onychomycosis Using Non-Thermal Plasma as a Prerequisite for Therapy. J Fungi (Basel) 2021;7(9):715. doi: 10.3390/jof7090715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta AK, Venkataraman M, Joshi LT, Cooper EA. Potential use of microwave technology in dermatology. J Dermatolog Treat. 2022;33(7):2899–2910. doi: 10.1080/09546634.2022.2089333. [DOI] [PubMed] [Google Scholar]
- 29.Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147(11):1277–1282. doi: 10.1001/archdermatol.2011.267. [DOI] [PubMed] [Google Scholar]
- 30.Gupta AK, Stec N. Recent advances in therapies for onychomycosis and its management. F1000Res. 2019;8 doi: 10.12688/f1000research.18646.1. F1000 Faculty Rev-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31(7):1111–1118. doi: 10.1111/jdv.14212. [DOI] [PubMed] [Google Scholar]
- 32.Rajbanshi B, Shen L, Jiang M, et al. Comparative Study of Traditional Ablative CO2 Laser-Assisted Topical Antifungal with only Topical Antifungal for Treating Onychomycosis: A Multicenter Study. Clin Drug Investig. 2020;40(6):575–582. doi: 10.1007/s40261-020-00914-6. [DOI] [PubMed] [Google Scholar]
- 33.Bhatta AK, Keyal U, Huang X, Zhao JJ. Fractional carbon-dioxide (CO2) laser-assisted topical therapy for the treatment of onychomycosis. J Am Acad Dermatol. 2016;74(5):916–923. doi: 10.1016/j.jaad.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Shi J, Li J, Huang H, et al. The efficacy of fractional carbon dioxide (CO2) laser combined with terbinafine hydrochloride 1% cream for the treatment of onychomycosis. J Cosmet Laser Ther. 2017;19(6):353–359. doi: 10.1080/14764172.2017.1334925. [DOI] [PubMed] [Google Scholar]
- 35.Lee SK, Kim HY, Lee JH, Lee UH, Kim MS. Real-world effectiveness of a fractional CO2 laser with topical antifungal agents for the treatment of onychomycosis. Dermatol Ther. 2022;35(6):e15498. doi: 10.1111/dth.15498. [DOI] [PubMed] [Google Scholar]
- 36.Han Y, Wang Y, Zhang XR, Chen J, Li XD. The effects of CO2 laser and topical agent combination therapy for onychomycosis: A meta-analysis. Dermatol Ther. 2021;34(6):e15136. doi: 10.1111/dth.15136. [DOI] [PubMed] [Google Scholar]
- 37.Ortner VK, Nguyen N, Brewer JR, Solovyeva V, Haedersdal M, Philipsen PA. Fractional CO2 laser ablation leads to enhanced permeation of a fluorescent dye in healthy and mycotic nails-An imaging investigation of laser-tissue effects and their impact on ungual drug delivery. Lasers Surg Med. 2022;54(6):861–874. doi: 10.1002/lsm.23541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Einarson TR. Pharmacoeconomic applications of meta-analysis for single groups using antifungal onychomycosis lacquers as an example. Clin Ther. 1997;19(3):559–569. doi: 10.1016/s0149-2918(97)80140-3. [DOI] [PubMed] [Google Scholar]
- 39.Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43(4 Suppl):S70–80. doi: 10.1067/mjd.2000.109071. [DOI] [PubMed] [Google Scholar]
- 40.Shemer A, Nathansohn N, Trau H, Amichai B, Grunwald MH. Ciclopirox nail lacquer for the treatment of onychomycosis: an open non-comparative study. J Dermatol. 2010;37(2):137–139. doi: 10.1111/j.1346-8138.2009.00773.x. [DOI] [PubMed] [Google Scholar]
- 41.Crawford F, Hollis S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst Rev. 2007;2007(3):CD001434. doi: 10.1002/14651858.CD001434.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hay RJ, Mackie RM, Clayton YM. Tioconazole nail solution--an open study of its efficacy in onychomycosis. Clin Exp Dermatol. 1985;10(2):111–115. doi: 10.1111/j.1365-2230.1985.tb00537.x. [DOI] [PubMed] [Google Scholar]


