Abstract
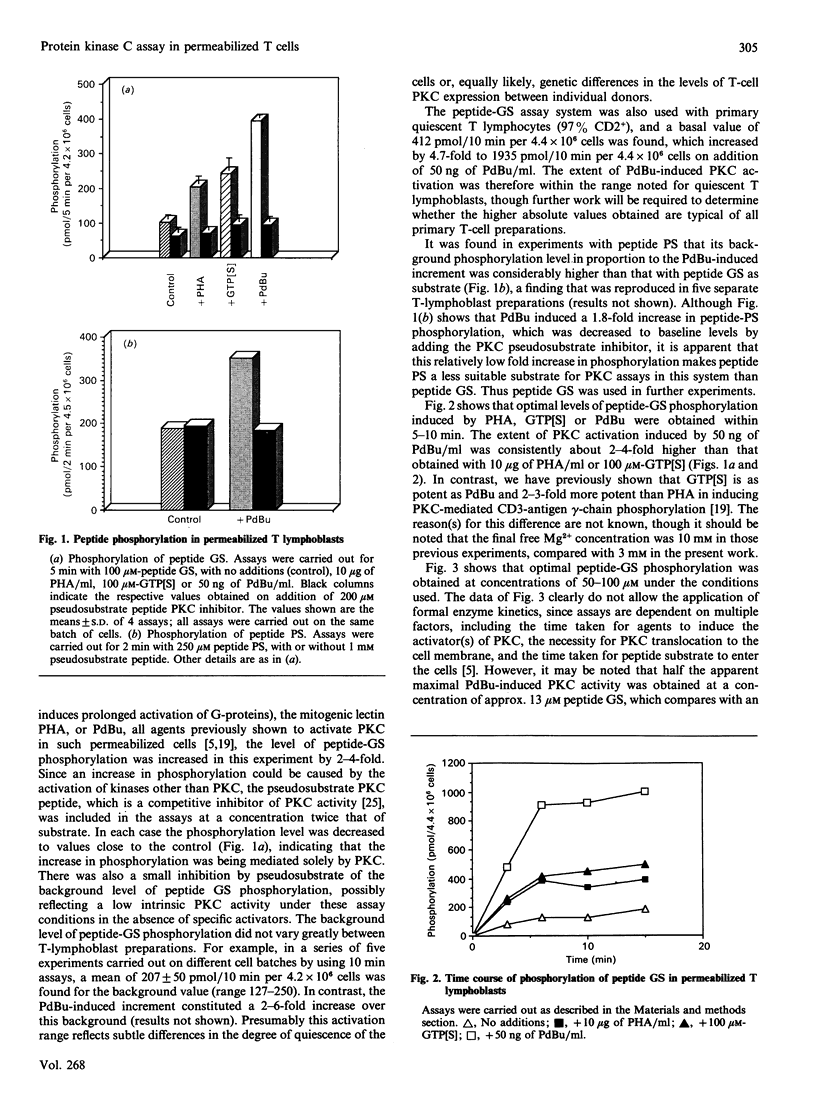
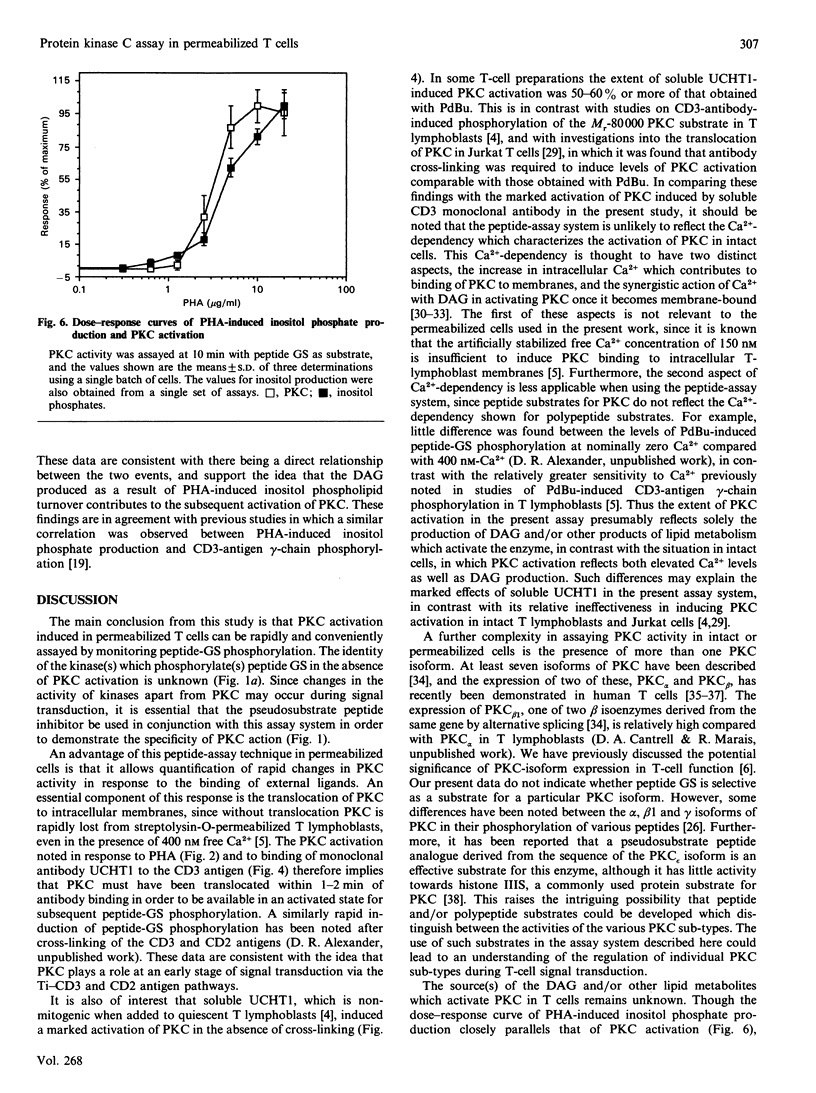
Activation of protein kinase C (PKC) in human T lymphocytes is an immediate consequence of mitogenic signalling via the antigen-receptor complex and CD2 antigen. In order to investigate further the signal-transduction pathways which result in PKC activation, we have established a novel PKC assay system using streptolysin-O-permeabilized T cells. Known peptide substrates of PKC were introduced into permeabilized cells in the presence of [gamma-32P]ATP, 3 mM-Mg2+ and 150 nM free Ca2+. The peptide found to have the lowest background phosphorylation had the sequence Pro-Leu-Ser-Arg-Thr-Leu-Ser-Val-Ala-Ala-Lys-Lys (peptide GS), and the phosphorylation of the peptide was increased up to 6-fold by direct activation of PKC with phorbol 12,13-dibutyrate. Induction of PKC activation with the UCHT1 antibody against the CD3 antigen, or with phytohaemagglutinin (PHA) or guanosine 5'-[gamma-thio]triphosphate (GTP[S]), increased peptide-GS phosphorylation by 2-3 fold. The specificity of PKC action on peptide GS was demonstrated by blocking increases in phosphorylation with a pseudosubstrate peptide PKC inhibitor. PKC activation by this technique could be detected within 1 min of adding external ligand. Dose-response curves revealed that PHA-induced production of inositol phosphates correlated closely with PKC activities, whereas only a partial correlation between these parameters was observed with GTP[S]. Our data are consistent with the presence of more than one G-protein-mediated pathway of PKC regulation in T cells. The quantitative PKC assay system described is both simple and reproducible, and its potential application to a wide range of cell types should prove useful in further investigations of PKC activation mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Cantrell D. A. Kinases and phosphatases in T-cell activation. Immunol Today. 1989 Jun;10(6):200–205. doi: 10.1016/0167-5699(89)90325-3. [DOI] [PubMed] [Google Scholar]
- Alexander D. R., Hexham J. M., Lucas S. C., Graves J. D., Cantrell D. A., Crumpton M. J. A protein kinase C pseudosubstrate peptide inhibits phosphorylation of the CD3 antigen in streptolysin-O-permeabilized human T lymphocytes. Biochem J. 1989 Jun 15;260(3):893–901. doi: 10.1042/bj2600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthes J. C., Eckel S., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D in homogenates from HL-60 granulocytes: implications of calcium and G protein control. Biochem Biophys Res Commun. 1989 Aug 30;163(1):657–664. doi: 10.1016/0006-291x(89)92187-6. [DOI] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Cantrell D. A., Davies A. A., Crumpton M. J. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Friedrich B., Davies A. A., Gullberg M., Crumpton M. J. Evidence that a kinase distinct from protein kinase C induces CD3 gamma-subunit phosphorylation without a concomitant down-regulation in CD3 antigen expression. J Immunol. 1989 Mar 1;142(5):1626–1630. [PubMed] [Google Scholar]
- Cantrell D., Davies A. A., Londei M., Feldman M., Crumpton M. J. Association of phosphorylation of the T3 antigen with immune activation of T lymphocytes. Nature. 1987 Feb 5;325(6104):540–542. doi: 10.1038/325540a0. [DOI] [PubMed] [Google Scholar]
- Cazaubon S., Marais R., Parker P., Strosberg A. D. Monoclonal antibodies to protein kinase C gamma. Functional relationship between epitopes and cofactor binding sites. Eur J Biochem. 1989 Jun 15;182(2):401–406. doi: 10.1111/j.1432-1033.1989.tb14845.x. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Davies A. A., Cantrell D. A., Hexham J. M., Parker P. J., Rothbard J., Crumpton M. J. The human T3 gamma chain is phosphorylated at serine 126 in response to T lymphocyte activation. J Biol Chem. 1987 Aug 15;262(23):10918–10921. [PubMed] [Google Scholar]
- Friedrich B., Cantrell D. A., Gullberg M. Evidences for protein kinase C. Activation in T lymphocytes by stimulation of either the CD2 or CD3 antigens. Eur J Immunol. 1989 Jan;19(1):17–23. doi: 10.1002/eji.1830190104. [DOI] [PubMed] [Google Scholar]
- Graves J. D., Lucas S. C., Alexander D. R., Cantrell D. A. Guanine nucleotide regulation of inositol phospholipid hydrolysis and CD3-antigen phosphorylation in permeabilized T lymphocytes. Biochem J. 1990 Jan 15;265(2):407–413. doi: 10.1042/bj2650407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- House C., Wettenhall R. E., Kemp B. E. The influence of basic residues on the substrate specificity of protein kinase C. J Biol Chem. 1987 Jan 15;262(2):772–777. [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J., Weyand C., Goronzy J. Antigen recognition by a human T cell clone leads to increases in inositol trisphosphate. J Immunol. 1987 Mar 1;138(5):1322–1324. [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Irving S. G., June C. H., Zipfel P. F., Siebenlist U., Kelly K. Mitogen-induced genes are subject to multiple pathways of regulation in the initial stages of T-cell activation. Mol Cell Biol. 1989 Mar;9(3):1034–1040. doi: 10.1128/mcb.9.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Tanaka T., Yoshida T., Onoda K., Ohta H., Hagiwara M., Itoh Y., Ogura M., Saito H., Hidaka H. Immunocytochemical evidence for translocation of protein kinase C in human megakaryoblastic leukemic cells: synergistic effects of Ca2+ and activators of protein kinase C on the plasma membrane association. J Cell Biol. 1988 Sep;107(3):929–937. doi: 10.1083/jcb.107.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky S. C., Loader J. E., Benedict S. H. Phorbol ester activation of phospholipase D in human monocytes but not peripheral blood lymphocytes. Biochem Biophys Res Commun. 1989 Jul 31;162(2):788–793. doi: 10.1016/0006-291x(89)92379-6. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Wahi M., Newton M. E., Weiss A. Heterogeneity of protein kinase C isoenzyme gene expression in human T cell lines. Protein kinase C-beta is not required for several T cell functions. J Immunol. 1989 Sep 1;143(5):1692–1695. [PubMed] [Google Scholar]
- Manger B., Weiss A., Imboden J., Laing T., Stobo J. D. The role of protein kinase C in transmembrane signaling by the T cell antigen receptor complex. Effects of stimulation with soluble or immobilized CD3 antibodies. J Immunol. 1987 Oct 15;139(8):2755–2760. [PubMed] [Google Scholar]
- Muramatsu M., Kaibuchi K., Arai K. A protein kinase C cDNA without the regulatory domain is active after transfection in vivo in the absence of phorbol ester. Mol Cell Biol. 1989 Feb;9(2):831–836. doi: 10.1128/mcb.9.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Olive D., Poggi A., Kozumbo W. J., Moretta L., Moretta A. Transmembrane signalling via the T11-dependent pathway of human T cell activation. Evidence for the involvement of 1,2-diacylglycerol and inositol phosphates. Eur J Immunol. 1987 Jan;17(1):55–60. doi: 10.1002/eji.1830170110. [DOI] [PubMed] [Google Scholar]
- Patel M. D., Samelson L. E., Klausner R. D. Multiple kinases and signal transduction. Phosphorylation of the T cell antigen receptor complex. J Biol Chem. 1987 Apr 25;262(12):5831–5838. [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Phosphorylation of an acidic mol. wt. 80 000 cellular protein in a cell-free system and intact Swiss 3T3 cells: a specific marker of protein kinase C activity. EMBO J. 1986 Jan;5(1):77–83. doi: 10.1002/j.1460-2075.1986.tb04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Harford J., Schwartz R. H., Klausner R. D. A 20-kDa protein associated with the murine T-cell antigen receptor is phosphorylated in response to activation by antigen or concanavalin A. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1969–1973. doi: 10.1073/pnas.82.7.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura S., Ase K., Berry N., Kikkawa U., McCaffrey P. G., Minowada J., Nishizuka Y. Expression of protein kinase C subspecies in human leukemia-lymphoma cell lines. FEBS Lett. 1989 Apr 24;247(2):353–357. doi: 10.1016/0014-5793(89)81369-9. [DOI] [PubMed] [Google Scholar]
- Schaap D., Parker P. J., Bristol A., Kriz R., Knopf J. Unique substrate specificity and regulatory properties of PKC-epsilon: a rationale for diversity. FEBS Lett. 1989 Jan 30;243(2):351–357. doi: 10.1016/0014-5793(89)80160-7. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Berry N., Oda T., Ase K., Kikkawa U., Nishizuka Y. Isolation of protein kinase C subspecies from a preparation of human T lymphocytes. FEBS Lett. 1988 Jul 18;234(2):387–391. doi: 10.1016/0014-5793(88)80122-4. [DOI] [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Ca2+ mobilization primes protein kinase C in human platelets. Ca2+ and phorbol esters stimulate platelet aggregation and secretion synergistically through protein kinase C. Biochem J. 1988 Oct 1;255(1):309–318. [PMC free article] [PubMed] [Google Scholar]
- Tettenborn C. S., Mueller G. C. 12-O-tetradecanoylphorbol-13-acetate activates phosphatidylethanol and phosphatidylglycerol synthesis by phospholipase D in cell lysates. Biochem Biophys Res Commun. 1988 Aug 30;155(1):249–255. doi: 10.1016/s0006-291x(88)81076-3. [DOI] [PubMed] [Google Scholar]
- Trilivas I., Brown J. H. Increases in intracellular Ca2+ regulate the binding of [3H]phorbol 12,13-dibutyrate to intact 1321N1 astrocytoma cells. J Biol Chem. 1989 Feb 25;264(6):3102–3107. [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Wallace D. L., Beverley P. C. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990 Mar;69(3):460–467. [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]



