Abstract
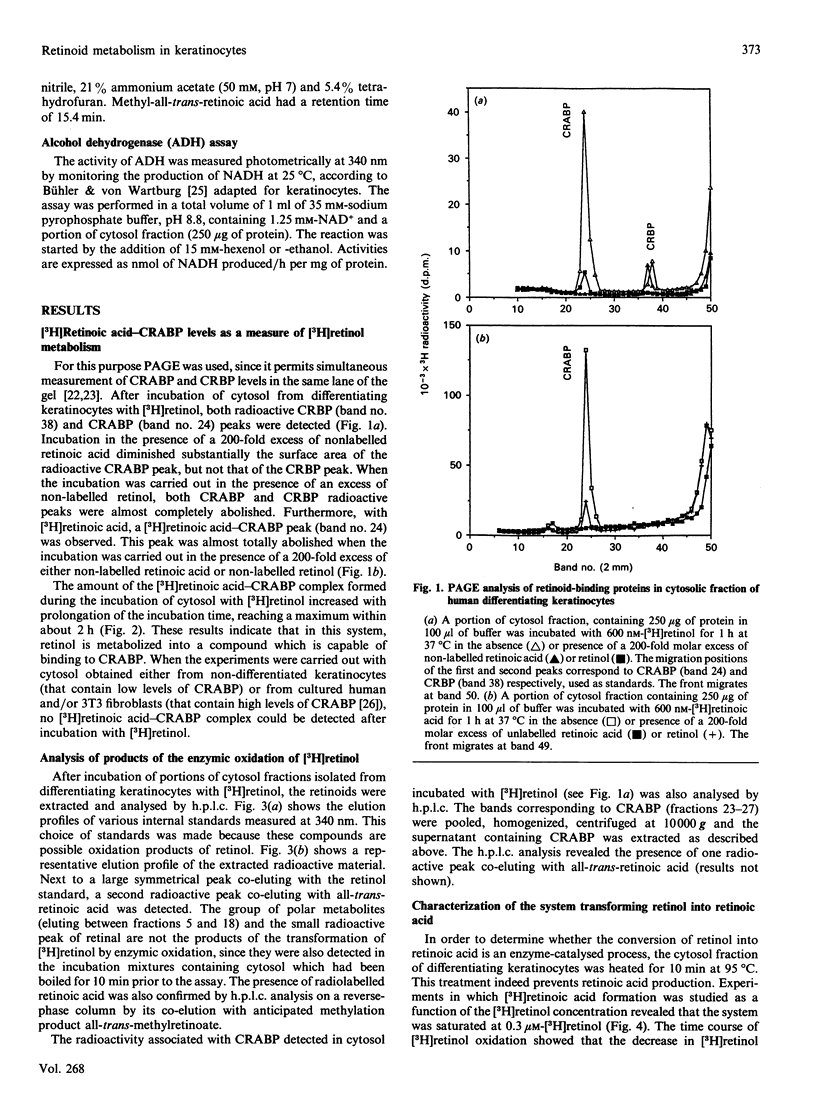
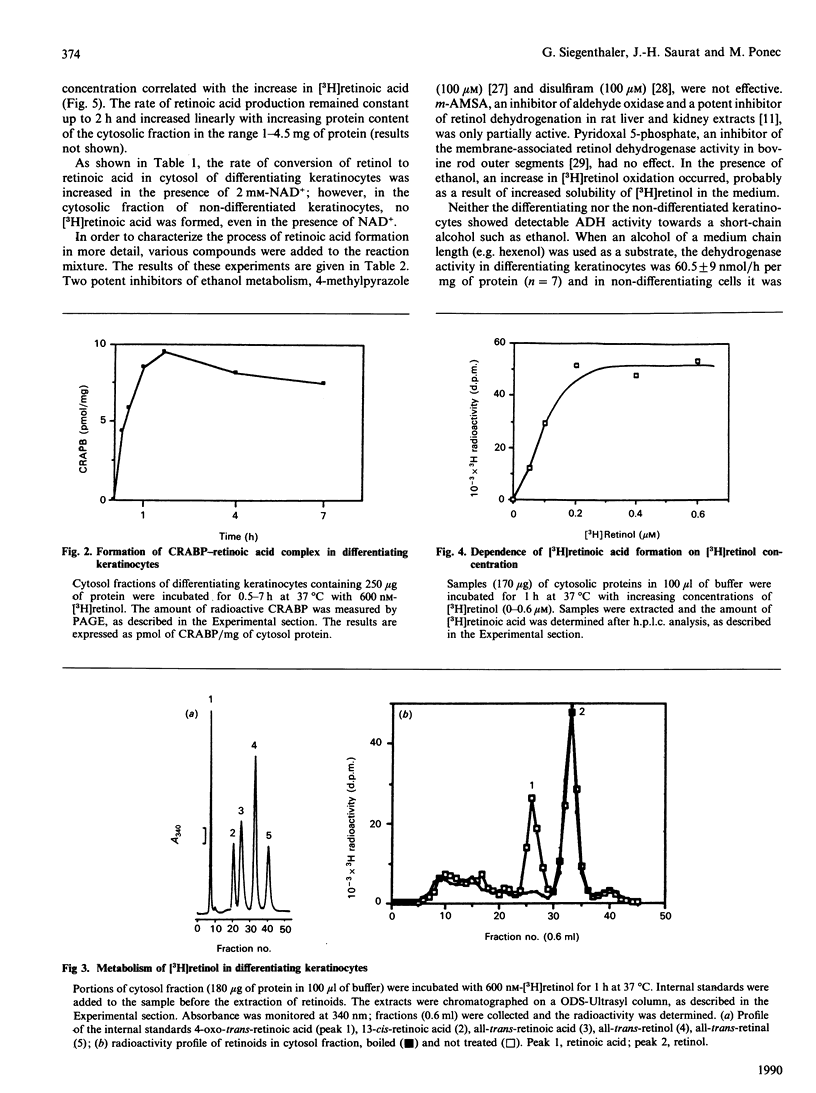
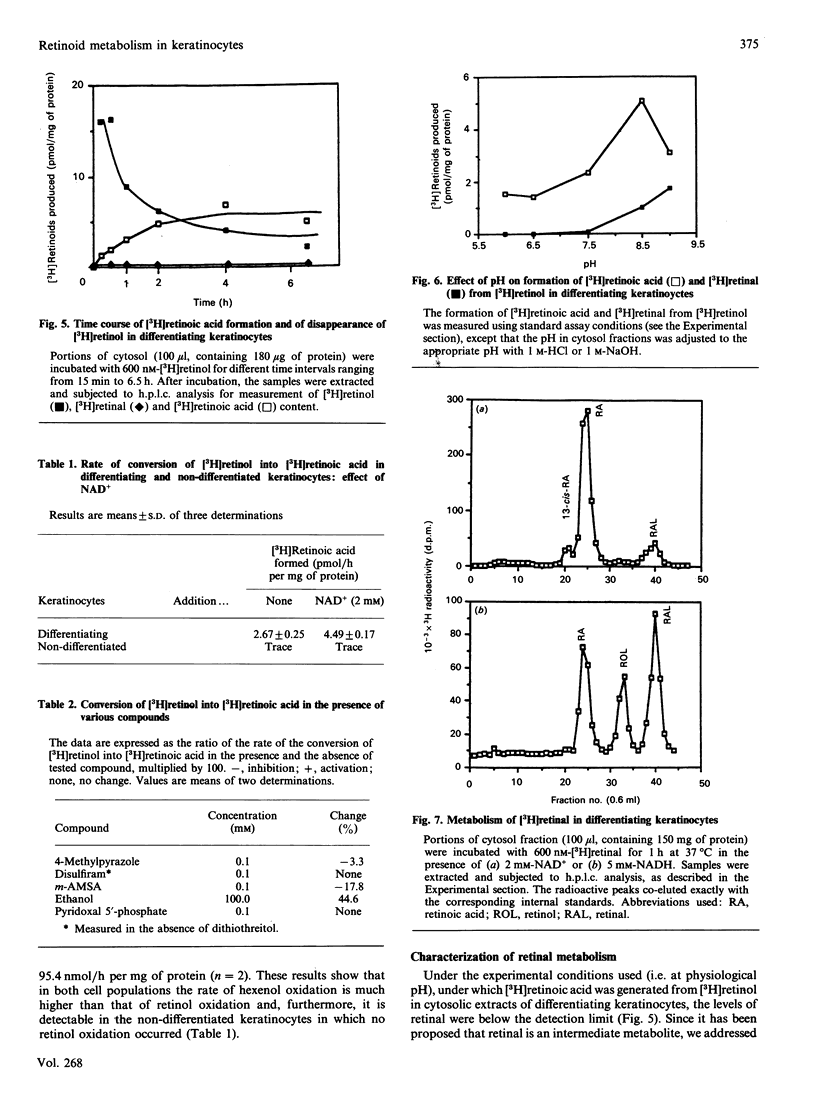
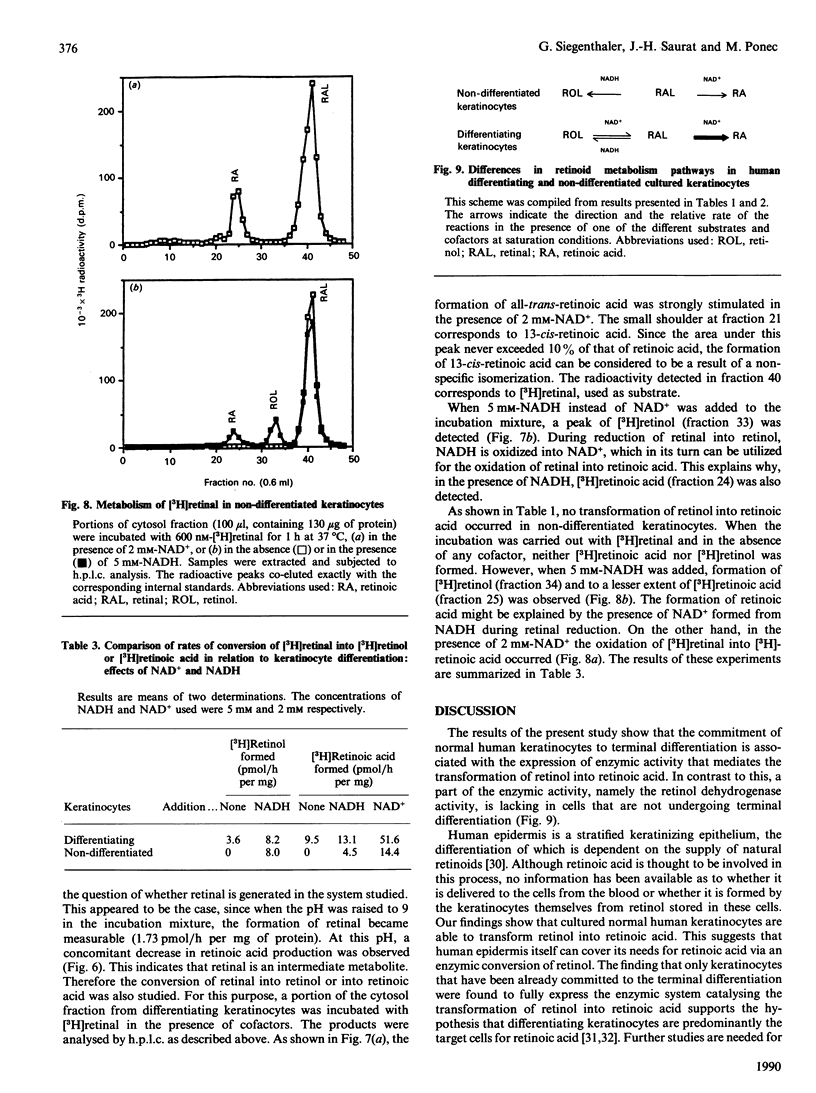
Cultured keratinocytes offer an attractive model for studying the metabolism of retinol in relation to cell differentiation, since the extent of keratinocyte differentiation can be modulated experimentally. The metabolism of retinol and retinal was studied in cytosol fractions prepared from two distinct keratinocyte populations, differentiating and non-differentiated. The enzymic activities were analysed using physiological concentrations of [3H]retinol and [3H]retinal in the presence of cofactors. The products formed were quantified by h.p.l.c. In the population of differentiating keratinocytes, the formation of retinoic acid from retinol occurred at a rate of 4.49 +/- 0.17 pmol/h per mg of protein, but no such conversion was observed in the population of non-differentiated cells. However, when retinal was used as substrate, retinoic acid was formed in both cell populations, at rates of 14.4 pmol/h per mg of protein in non-differentiated and 51.6 pmol/h per mg of protein in differentiating keratinocytes. Using PAGE/radiobinding assay, we demonstrated that retinoic acid formed from retinol was bound in differentiating keratinocytes to endogenous cellular retinoic acid-binding protein (CRABP). Furthermore, retinal was reduced to retinol in the presence of NADH in both differentiating and non-differentiated keratinocytes at a similar rate (8 pmol/h per mg of protein). Although retinal could not be detected under physiological conditions, it was found in significant amounts at pH 8.5-9, which is optimal for enzymic activity. This indicates that in keratinocytes retinal is an intermediate metabolite in retinoic acid formation from retinol. The enzymes catalysing the conversion of retinol into retinoic acid were found to differ from other alcohol and aldehyde dehydrogenases, since the formation of retinoic acid was not significantly affected by specific inhibitors of alcohol metabolism, such as 4-methylpyrazole and disulfiram. Moreover, the cytosol of non-differentiated keratinocytes did not generate retinoic acid from retinol despite showing alcohol dehydrogenase activity. The results suggest that: (1) retinol metabolism in human keratinocytes is different from that of other alcohols, (2) retinal is an intermediate metabolite in the conversion of retinol into retinoic acid, and (3) differentiating keratinocytes rich in CRABP are probably target cells for retinoic acid action.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselineau D., Bernard B. A., Bailly C., Darmon M. Retinoic acid improves epidermal morphogenesis. Dev Biol. 1989 Jun;133(2):322–335. doi: 10.1016/0012-1606(89)90037-7. [DOI] [PubMed] [Google Scholar]
- Ball S., Goodwin T. W., Morton R. A. Studies on vitamin A: 5. The preparation of retinene(1)-vitamin A aldehyde. Biochem J. 1948;42(4):516–523. doi: 10.1042/bj0420516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner W. S., Churchich J. E. The membrane bound retinol dehydrogenase from bovine rod outer segments. Biochem Biophys Res Commun. 1980 Jun 16;94(3):820–826. doi: 10.1016/0006-291x(80)91308-x. [DOI] [PubMed] [Google Scholar]
- Brennan J. K., Mansky J., Roberts G., Lichtman M. A. Improved methods for reducing calcium and magnesium concentrations in tissue culture medium: application to studies of lymphoblast proliferation in vitro. In Vitro. 1975 Nov-Dec;11(6):354–360. doi: 10.1007/BF02616371. [DOI] [PubMed] [Google Scholar]
- Bühler R., von Wartburg J. P. Purification and substrate specificities of three human liver alcohol dehydrogenase isoenzymes. FEBS Lett. 1982 Jul 19;144(1):135–139. doi: 10.1016/0014-5793(82)80586-3. [DOI] [PubMed] [Google Scholar]
- Connor M. J., Smit M. H. Terminal-group oxidation of retinol by mouse epidermis. Inhibition in vitro and in vivo. Biochem J. 1987 Jun 1;244(2):489–492. doi: 10.1042/bj2440489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M. J., Smit M. H. The formation of all-trans-retinoic acid from all-trans-retinol in hairless mouse skin. Biochem Pharmacol. 1987 Mar 15;36(6):919–924. doi: 10.1016/0006-2952(87)90185-7. [DOI] [PubMed] [Google Scholar]
- DOWLING J. E., WALD G. The role of vitamin A acid. Vitam Horm. 1960;18:515–541. doi: 10.1016/s0083-6729(08)60878-x. [DOI] [PubMed] [Google Scholar]
- De Leenheer A. P., Lambert W. E., Claeys I. All-trans-retinoic acid: measurement of reference values in human serum by high performance liquid chromatography. J Lipid Res. 1982 Dec;23(9):1362–1367. [PubMed] [Google Scholar]
- Goedde H. W., Agarwal D. P., Eckey R., Harada S. Population genetic and family studies on aldehyde dehydrogenase deficiency and alcohol sensitivity. Alcohol. 1985 May-Jun;2(3):383–390. doi: 10.1016/0741-8329(85)90099-0. [DOI] [PubMed] [Google Scholar]
- Goedde H. W., Agarwal D. P., Harada S. Alcohol metabolizing enzymes: studies of isozymes in human biopsies and cultured fibroblasts. Clin Genet. 1979 Jul;16(1):29–33. doi: 10.1111/j.1399-0004.1979.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978 Nov;15(3):801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Koen A. L., Shaw C. R. Retinol and alcohol dehydrogenases in retina and liver. Biochim Biophys Acta. 1966 Oct 17;128(1):48–54. doi: 10.1016/0926-6593(66)90140-8. [DOI] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakshman M. R., Mychkovsky I., Attlesey M. Enzymatic conversion of all-trans-beta-carotene to retinal by a cytosolic enzyme from rabbit and rat intestinal mucosa. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9124–9128. doi: 10.1073/pnas.86.23.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M., Ong D. E., Summerbell D., Chytil F. Spatial distribution of cellular protein binding to retinoic acid in the chick limb bud. Nature. 1988 Oct 20;335(6192):733–735. doi: 10.1038/335733a0. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Nyberg J. A., Bodin N. O., Hansson E. Toxicity of pyrazole and 4-methylpyrazole in mice and rats. Experientia. 1972 Oct 15;28(10):1198–1200. doi: 10.1007/BF01946169. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Race K. R. Biogenesis of retinoic acid from beta-carotene. Differences between the metabolism of beta-carotene and retinal. J Biol Chem. 1988 Nov 25;263(33):17372–17377. [PubMed] [Google Scholar]
- Napoli J. L., Race K. R. The biosynthesis of retinoic acid from retinol by rat tissues in vitro. Arch Biochem Biophys. 1987 May 15;255(1):95–101. doi: 10.1016/0003-9861(87)90298-0. [DOI] [PubMed] [Google Scholar]
- Napoli J. L. Retinol metabolism in LLC-PK1 Cells. Characterization of retinoic acid synthesis by an established mammalian cell line. J Biol Chem. 1986 Oct 15;261(29):13592–13597. [PubMed] [Google Scholar]
- Posch K. C., Enright W. J., Napoli J. L. Retinoic acid synthesis by cytosol from the alcohol dehydrogenase negative deermouse. Arch Biochem Biophys. 1989 Oct;274(1):171–178. doi: 10.1016/0003-9861(89)90428-1. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977 Feb 3;265(5593):421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Siegenthaler G., Saurat J. H. A slab gel electrophoresis technique for measurement of plasma retinol-binding protein, cellular retinol-binding and retinoic-acid-binding proteins in human skin. Eur J Biochem. 1987 Jul 1;166(1):209–214. doi: 10.1111/j.1432-1033.1987.tb13503.x. [DOI] [PubMed] [Google Scholar]
- Siegenthaler G., Saurat J. H., Ponec M. Terminal differentiation in cultured human keratinocytes is associated with increased levels of cellular retinoic acid-binding protein. Exp Cell Res. 1988 Sep;178(1):114–126. doi: 10.1016/0014-4827(88)90383-7. [DOI] [PubMed] [Google Scholar]
- Siegenthaler G., Saurat J. H. Retinoid binding proteins and human skin. Pharmacol Ther. 1989;40(1):45–54. doi: 10.1016/0163-7258(89)90073-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- THOMPSON J. N., HOWELL J. M., PITT G. A. VITAMIN A AND REPRODUCTION IN RATS. Proc R Soc Lond B Biol Sci. 1964 Feb 18;159:510–535. doi: 10.1098/rspb.1964.0017. [DOI] [PubMed] [Google Scholar]
- Takase S., Ong D. E., Chytil F. Transfer of retinoic acid from its complex with cellular retinoic acid-binding protein to the nucleus. Arch Biochem Biophys. 1986 Jun;247(2):328–334. doi: 10.1016/0003-9861(86)90591-6. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Characterization of retinoid metabolism in the developing chick limb bud. Development. 1988 Jul;103(3):473–483. doi: 10.1242/dev.103.3.473. [DOI] [PubMed] [Google Scholar]
- Thompson J. N., Howell J. M., Pitt G. A., McLaughlin C. I. The biological activity of retinoic acid in the domestic fowl and the effects of vitamin A deficiency on the chick embryo. Br J Nutr. 1969 Aug;23(3):471–490. doi: 10.1079/bjn19690056. [DOI] [PubMed] [Google Scholar]
- Vahlquist A., Lee J. B., Michaëlsson G., Rollman O. Vitamin A in human skin: II Concentrations of carotene, retinol and dehydroretinol in various components of normal skin. J Invest Dermatol. 1982 Aug;79(2):94–97. doi: 10.1111/1523-1747.ep12500033. [DOI] [PubMed] [Google Scholar]
- Vallari R. C., Pietruszko R. Human aldehyde dehydrogenase: mechanism of inhibition of disulfiram. Science. 1982 May 7;216(4546):637–639. doi: 10.1126/science.7071604. [DOI] [PubMed] [Google Scholar]
- Williams J. B., Napoli J. L. Metabolism of retinoic acid and retinol during differentiation of F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4658–4662. doi: 10.1073/pnas.82.14.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G. Multiple functions of vitamin A. Physiol Rev. 1984 Jul;64(3):873–937. doi: 10.1152/physrev.1984.64.3.873. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Lichti U. Regulation of epidermal transglutaminase activity and terminal differentiation by retinoids and phorbol esters. Cancer Res. 1983 Dec;43(12 Pt 1):5707–5712. [PubMed] [Google Scholar]
- Yuspa S. H., Lichti U., Ben T., Hennings H. Modulation of terminal differentiation and responses to tumor promoters by retinoids in mouse epidermal cell cultures. Ann N Y Acad Sci. 1981 Feb 27;359:260–273. doi: 10.1111/j.1749-6632.1981.tb12752.x. [DOI] [PubMed] [Google Scholar]


