Abstract
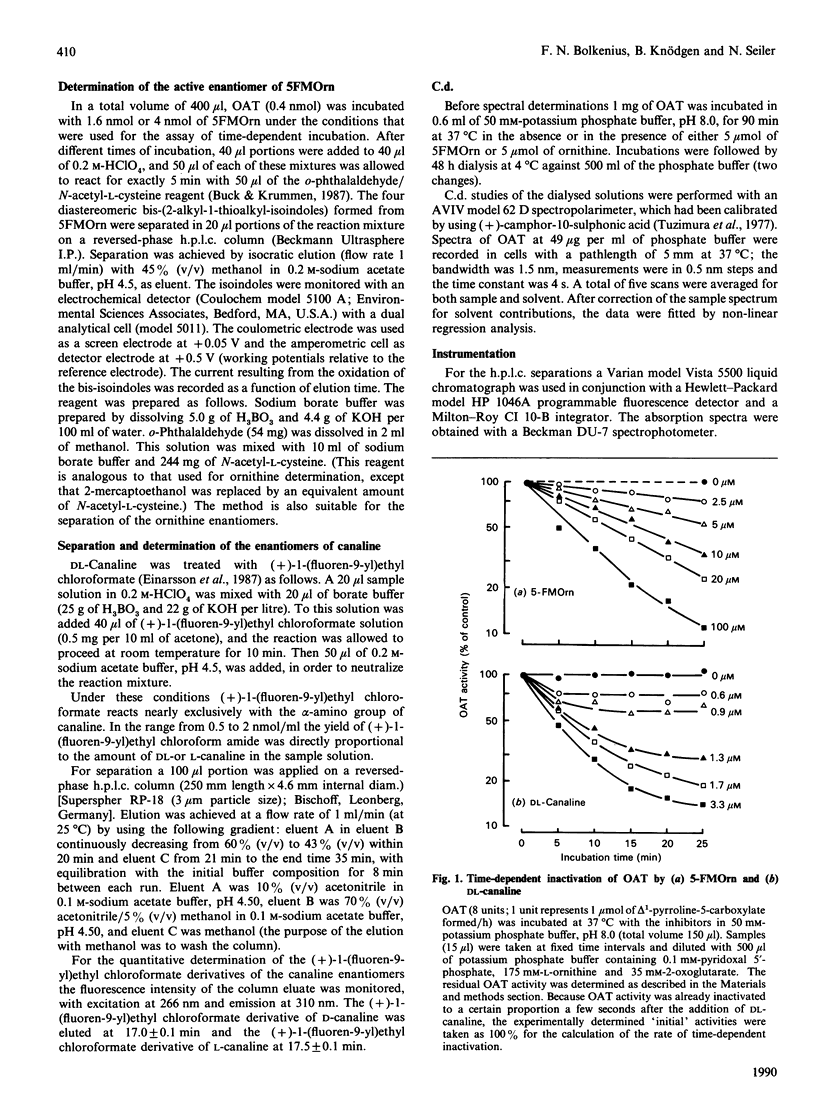
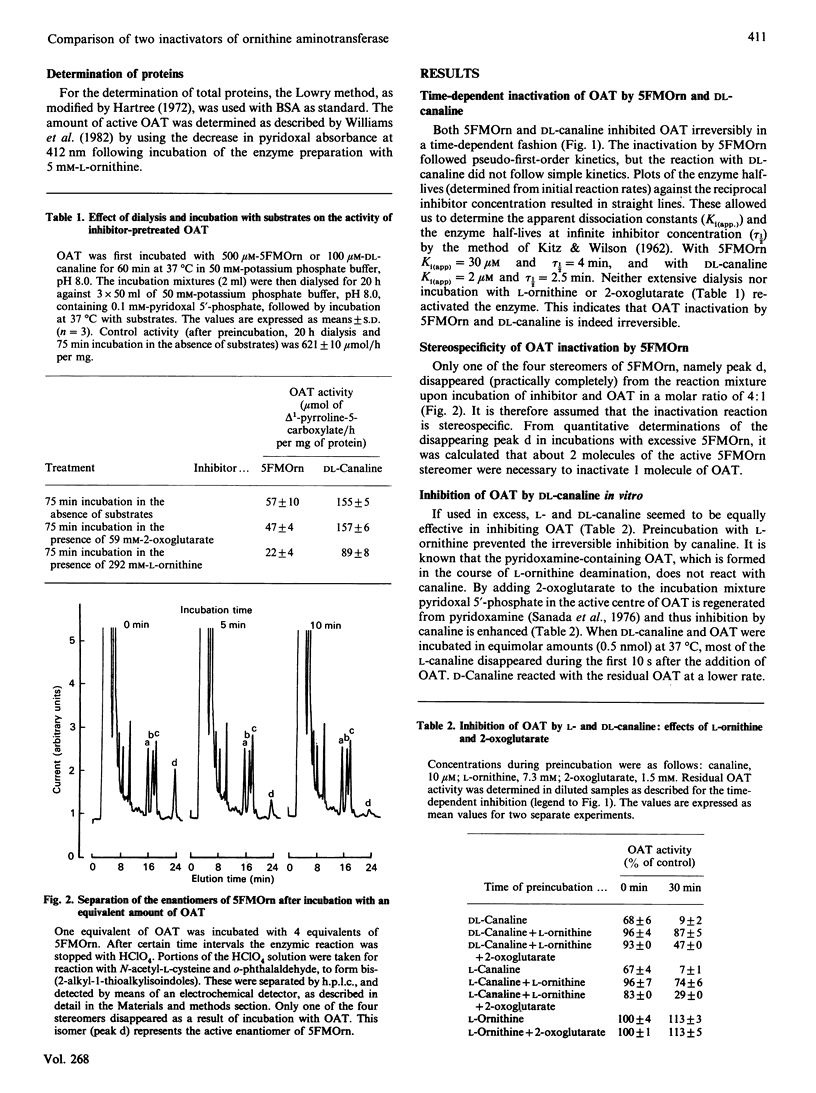
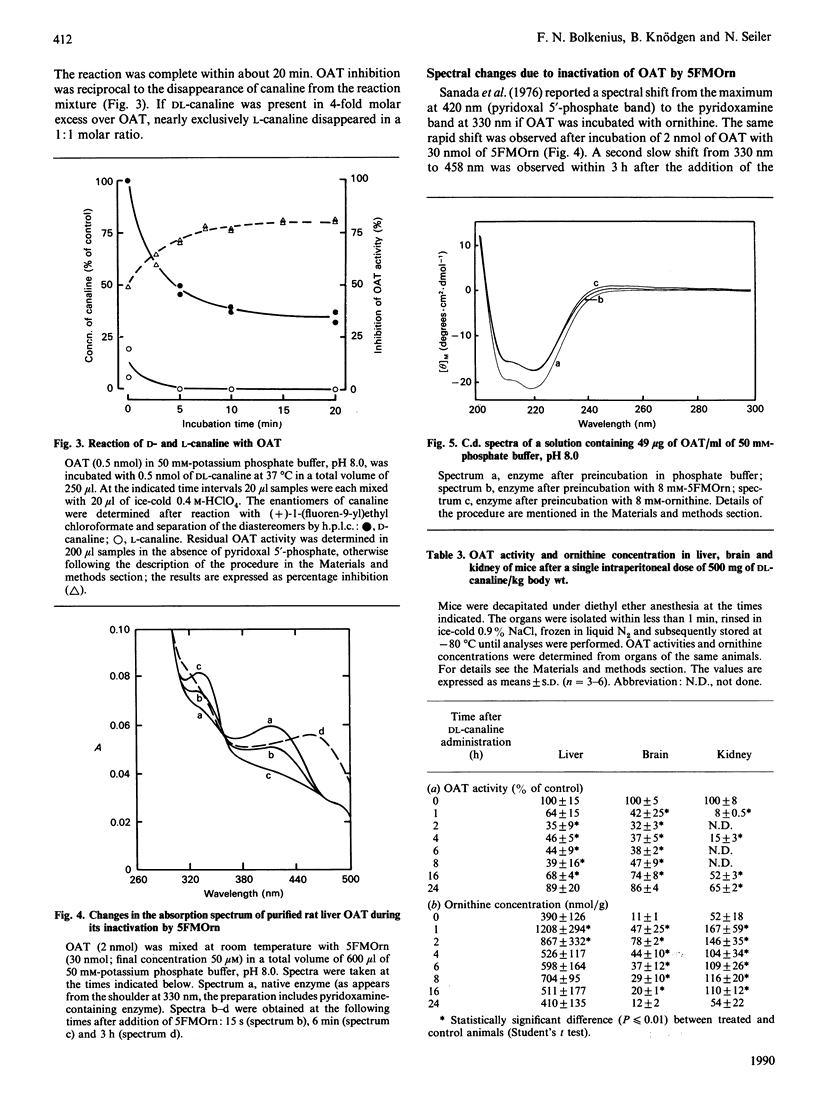
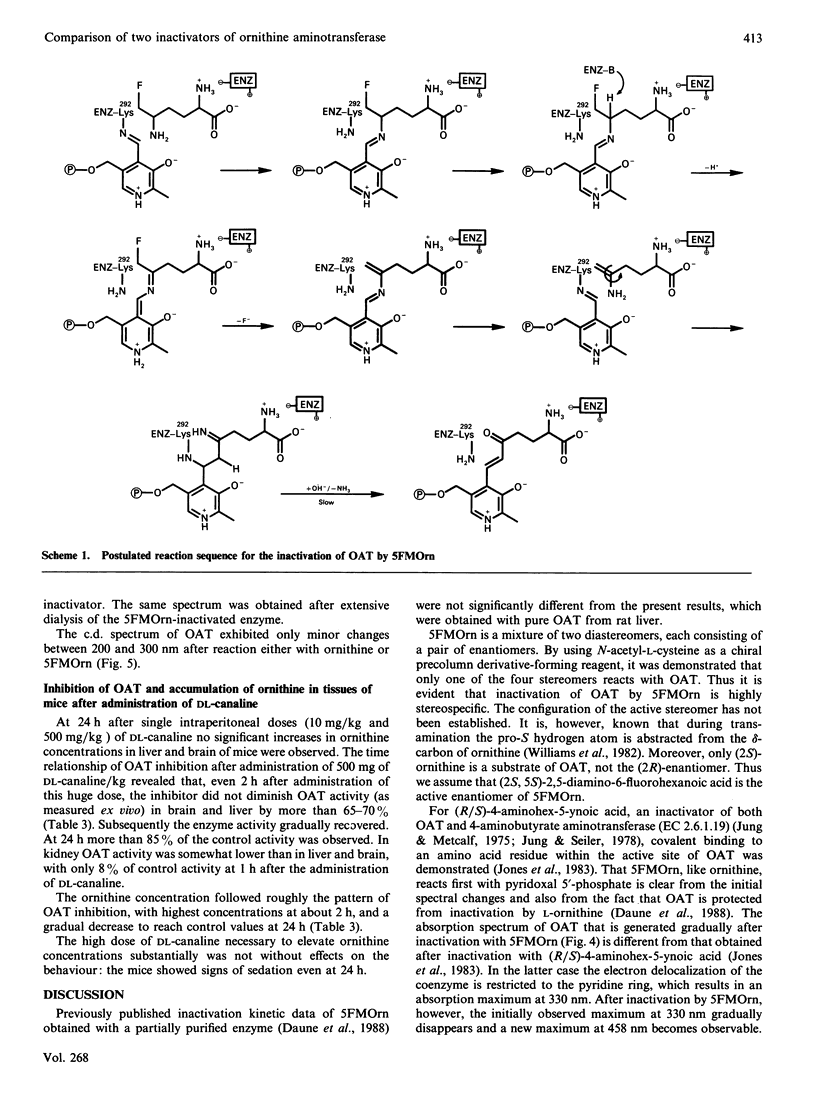
5-Fluoromethylornithine (5FMOrn) is an enzyme-activated irreversible inhibitor or ornithine aminotransferase (L-ornithine:2-oxo-acid 5-aminotransferase, OAT). For purified rat liver OAT, Ki(app.) was found to be 30 microM. and tau 1/2 = 4 min. Of the four stereomers of 5FMOrn only one reacts with OAT. The formation of a chromophore with an absorption maximum at 458 nm after inactivation of OAT by 5FMOrn suggests the formation of an enamine intermediate, which is slowly hydrolysed to release an unsaturated ketone. L-Canaline [(S)-2-amino-4-amino-oxybutyric acid] is a well-known irreversible inhibitor of OAT. Not only the natural L-enantiomer but also the D-enantiomer reacts by oxime formation with pyridoxal 5'-phosphate in the active site of the enzyme, although considerably more slowly. This demonstrates that the stereochemistry at C-2 of ornithine is not absolutely stringent. In vitro, canaline reacted faster than 5FMOrn with OAT. In vivo, however, only incomplete OAT inhibition was observed with canaline. Whereas intraperitoneal administration of 10 mg of 5FMOrn/kg body wt. to mice was sufficient to inactivate OAT in brain and liver by 90% for 24 h, 500 mg of DL-canaline/kg body wt. only produced a transient inhibition of 65-70%. The accumulation of ornithine in these tissues was considerably slower and the maximum concentrations lower than were achieved with 5FMOrn. It appears that DL-canaline, in contrast with 5FMOrn, is not useful as a tool in studies of biological consequences of OAT inhibition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck R. H., Krummen K. High-performance liquid chromatographic determination of enantiomeric amino acids and amino alcohols after derivatization with o-phthaldialdehyde and various chiral mercaptans. Application to peptide hydrolysates. J Chromatogr. 1987 Jan 30;387:255–265. doi: 10.1016/s0021-9673(01)94529-7. [DOI] [PubMed] [Google Scholar]
- Daune G., Gerhart F., Seiler N. 5-Fluoromethylornithine, an irreversible and specific inhibitor of L-ornithine:2-oxo-acid aminotransferase. Biochem J. 1988 Jul 15;253(2):481–488. doi: 10.1042/bj2530481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson S., Josefsson B., Möller P., Sanchez D. Separation of amino acid enantiomers and chiral amines using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase liquid chromatography. Anal Chem. 1987 Apr 15;59(8):1191–1195. doi: 10.1021/ac00135a025. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Jones E. D., Basford J. M., John R. A. An investigation of the properties of ornithine aminotransferase after inactivation by the 'suicide' inhibitor aminohexynoate and use of the compound as a probe of intracellullar protein turnover. Biochem J. 1983 Jan 1;209(1):243–249. doi: 10.1042/bj2090243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. J., Metcalf B. W. Catalytic inhibition of gamma-aminobutyric acid - alpha-ketoglutarate transaminase of bacterial origin by 4-aminohex-5-ynoic acid, a substrate analog. Biochem Biophys Res Commun. 1975 Nov 3;67(1):301–306. doi: 10.1016/0006-291x(75)90316-2. [DOI] [PubMed] [Google Scholar]
- Jung M. J., Seiler N. Enzyme-activated irreversible inhibitors of L-ornithine:2-oxoacid aminotransferase. Demonstration of mechanistic features of the inhibition of ornithine aminotransferase by 4-aminohex-5-ynoic acid and gabaculine and correlation with in vivo activity. J Biol Chem. 1978 Oct 25;253(20):7431–7439. [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Kito K., Sanada Y., Katunuma N. Mode of inhibition of ornithine aminotransferase by L-canaline. J Biochem. 1978 Jan;83(1):201–206. doi: 10.1093/oxfordjournals.jbchem.a131892. [DOI] [PubMed] [Google Scholar]
- McCown T. J., Givens B. S., Breese G. R. Amino acid influences on seizures elicited within the inferior colliculus. J Pharmacol Exp Ther. 1987 Nov;243(2):603–608. [PubMed] [Google Scholar]
- Peraino C., Bunville L. G., Tahmisian T. N. Chemical, physical, and morphological properties of ornithine Aminotransferase from rat liver. J Biol Chem. 1969 May 10;244(9):2241–2249. [PubMed] [Google Scholar]
- Rosenthal G. A. The biological and biochemical properties of L-canaline, a naturally occurring structural analogue of L-ornithine. Life Sci. 1978 Jul 10;23(2):93–98. doi: 10.1016/0024-3205(78)90255-2. [DOI] [PubMed] [Google Scholar]
- STRECKER H. J. PURIFICATION AND PROPERTIES OF RAT LIVER ORNITHINE DELTA-TRANSAMINASE. J Biol Chem. 1965 Mar;240:1225–1230. [PubMed] [Google Scholar]
- Seiler N., Daune G., Bolkenius F. N., Knödgen B. Ornithine aminotransferase activity, tissue ornithine concentrations and polyamine metabolism. Int J Biochem. 1989;21(4):425–432. doi: 10.1016/0020-711x(89)90367-4. [DOI] [PubMed] [Google Scholar]
- Silverman R. B., Invergo B. J. Mechanism of inactivation of gamma-aminobutyrate aminotransferase by 4-amino-5-fluoropentanoic acid. First example of an enamine mechanism for a gamma-amino acid with a partition ratio of 0. Biochemistry. 1986 Nov 4;25(22):6817–6820. doi: 10.1021/bi00370a013. [DOI] [PubMed] [Google Scholar]
- Silverman R. B., Levy M. A. Mechanism of inactivation of gamma-aminobutyric acid-alpha-ketoglutaric acid aminotransferase by 4-amino-5-halopentanoic acids. Biochemistry. 1981 Mar 3;20(5):1197–1203. doi: 10.1021/bi00508a022. [DOI] [PubMed] [Google Scholar]
- Simmaco M., John R. A., Barra D., Bossa F. The primary structure of ornithine aminotransferase. Identification of active-site sequence and site of post-translational proteolysis. FEBS Lett. 1986 Apr 7;199(1):39–42. doi: 10.1016/0014-5793(86)81219-4. [DOI] [PubMed] [Google Scholar]
- Tuzimura K., Konno T., Meguro H., Hatano M., Murakami T. A critical study of the measurement and calibration of circular dichroism. Anal Biochem. 1977 Jul;81(1):167–174. doi: 10.1016/0003-2697(77)90610-8. [DOI] [PubMed] [Google Scholar]
- Ueno H., Likos J. J., Metzler D. E. Chemistry of the inactivation of cytosolic aspartate aminotransferase by serine O-sulfate. Biochemistry. 1982 Aug 31;21(18):4387–4393. doi: 10.1021/bi00261a030. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Bridge G., Fowler L. J., John R. A. The reaction of ornithine aminotransferase with ornithine. Biochem J. 1982 Jan 1;201(1):221–225. doi: 10.1042/bj2010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski J. T., Blaker W. D., Meek J. L. Ornithine as a precursor of neurotransmitter glutamate: effect of canaline on ornithine aminotransferase activity and glutamate content in the septum of rat brain. Brain Res. 1985 Mar 11;329(1-2):161–168. doi: 10.1016/0006-8993(85)90521-9. [DOI] [PubMed] [Google Scholar]


