Abstract
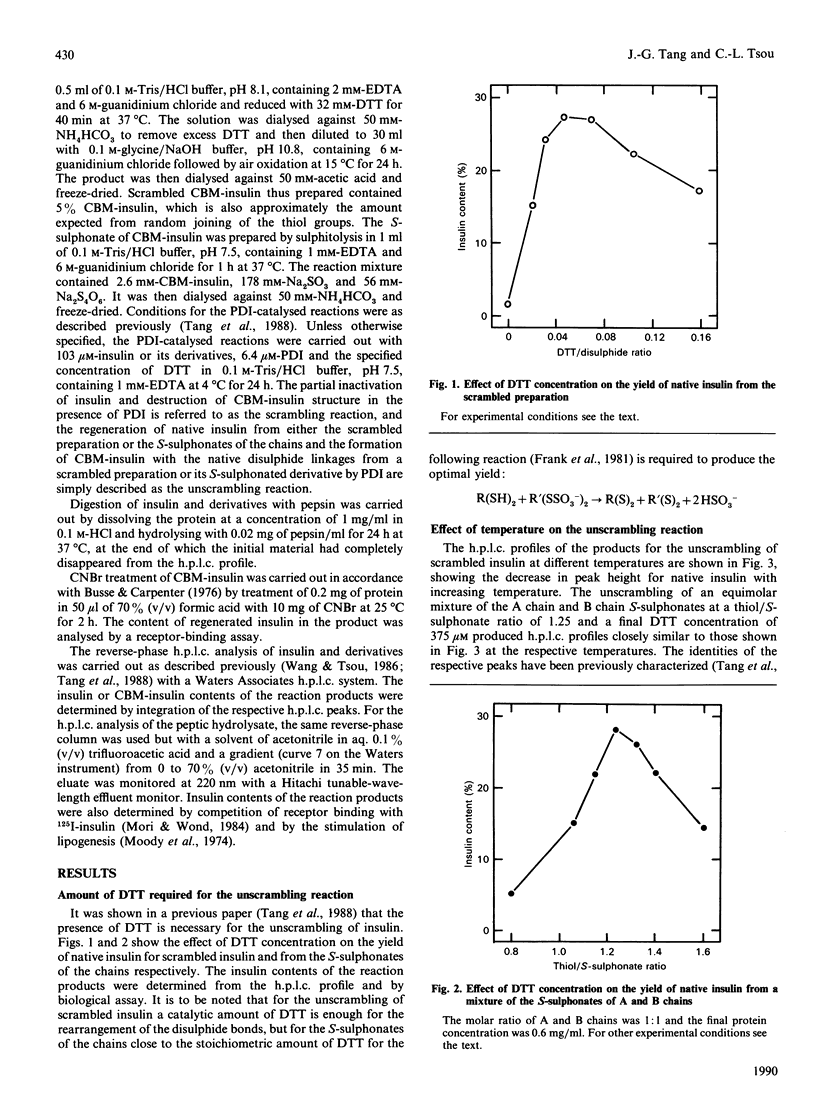
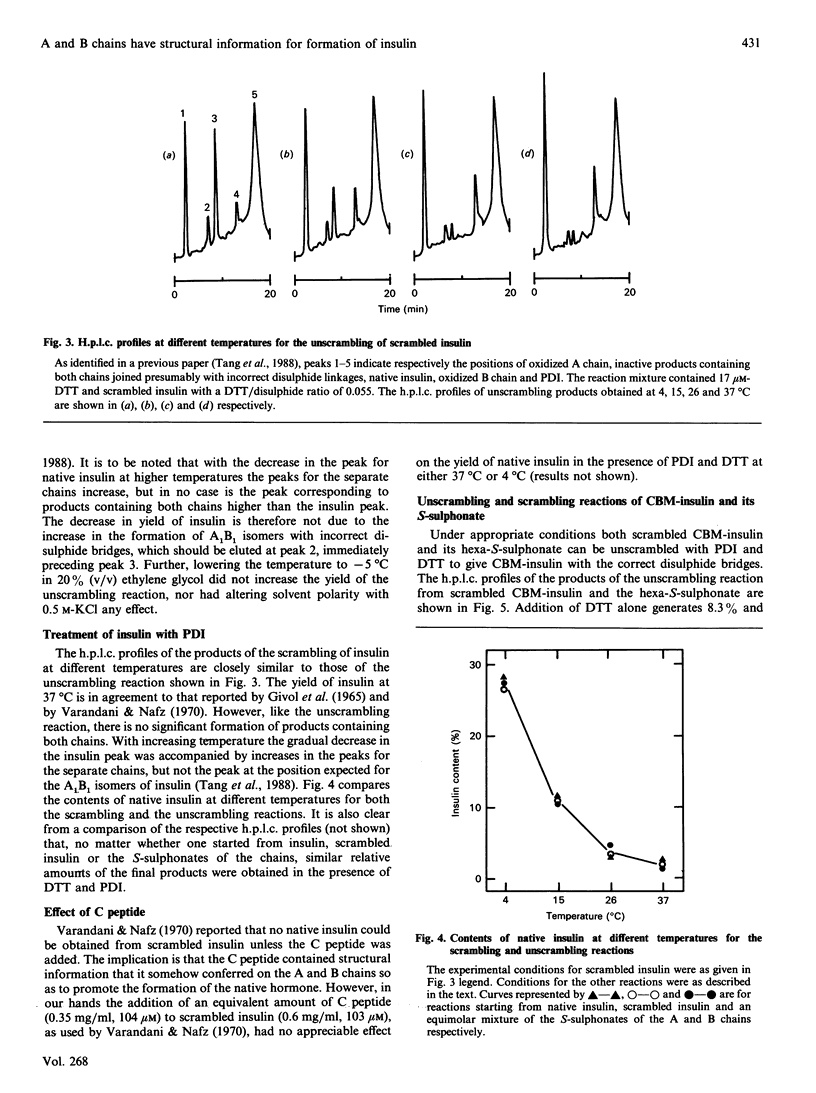
It has been shown previously [Tang, Wang & Tsou (1988) Biochem. J. 255, 451-455] that, under appropriate conditions, native insulin can be obtained from scrambled insulin or the S-sulphonates of the chains with a yield of 25-30%, together with reaction products containing the separated A and B chains. The native hormone is by far the predominant product among the isomers containing both chains. It is now shown that the presence of added C peptide has no appreciable effect on the yield of native insulin. At higher temperatures the content of the native hormone decreases whereas those of the separated chains increase, and in no case was scrambled insulin containing both chains the predominant product in the absence of denaturants. Both the scrambling and the unscrambling reactions give similar h.p.l.c. profiles for the products. Under similar conditions cross-linked insulin with native disulphide linkages can be obtained from the scrambled molecule or from the S-sulphonate derivative with yields of 50% and 75% respectively at 4 degrees C, and with a dilute solution of the hexa-S-sulphonate yields better than 90% can be obtained. The regenerated product is shown to have the native disulphide bridges by treatment with CNBr to give insulin and by the identity of the h.p.l.c. profile of its peptic hydrolysate with that for cross-linked insulin. It appears that the insulin A and B chains contain sufficient information for the formation of the native molecule and that the role of the connecting C peptide is to bring and to keep the two chains together.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B., Scheraga H. A. Experimental and theoretical aspects of protein folding. Adv Protein Chem. 1975;29:205–300. doi: 10.1016/s0065-3233(08)60413-1. [DOI] [PubMed] [Google Scholar]
- Brandenburg D., Gattner H. G., Schermutzki W., Schüttler A., Uschkoreit J., Weimann J., Wollmer A. Crosslinked insulins: preparation, properties, and application. Adv Exp Med Biol. 1977;86A:261–282. doi: 10.1007/978-1-4684-3282-4_16. [DOI] [PubMed] [Google Scholar]
- Brandenburg D., Wollmer A. The effect of a non-peptide interchain crosslink on the reoxidation of reduced insulin. Hoppe Seylers Z Physiol Chem. 1973 Jun;354(6):613–627. doi: 10.1515/bchm2.1973.354.1.613. [DOI] [PubMed] [Google Scholar]
- Busse W. D., Carpenter F. H. Synthesis and properties of carbonylbis(methionyl)insulin, a proinsulin analogue which is convertible to insulin by cyanogen bromide cleavage. Biochemistry. 1976 Apr 20;15(8):1649–1657. doi: 10.1021/bi00653a010. [DOI] [PubMed] [Google Scholar]
- Büllesbach E. E., Danho W., Helbig H. J., Zahn H. Human proinsulin, VIII: studies on the S-tritylation of reduced proinsulin, insulin A and B chains and their detritylation. Hoppe Seylers Z Physiol Chem. 1980;361(6):865–873. doi: 10.1515/bchm2.1980.361.1.865. [DOI] [PubMed] [Google Scholar]
- DU Y. C., ZHANG Y. S., LU Z. X., TSOU C. L. Resynthesis of insulin from its glycyl and phenylalanyl chains. Sci Sin. 1961 May;10:84–104. [PubMed] [Google Scholar]
- GIVOL D., DELORENZO F., GOLDBERGER R. F., ANFINSEN C. B. DISULFIDE INTERCHANGE AND THE THREE-DIMENSIONAL STRUCTURE OF PROTEINS. Proc Natl Acad Sci U S A. 1965 Mar;53:676–684. doi: 10.1073/pnas.53.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillson D. A., Lambert N., Freedman R. B. Formation and isomerization of disulfide bonds in proteins: protein disulfide-isomerase. Methods Enzymol. 1984;107:281–294. doi: 10.1016/0076-6879(84)07018-x. [DOI] [PubMed] [Google Scholar]
- Katsoyannis P. G., Trakatellis A. C., Johnson S., Zalut C., Schwartz G. Studies on the synthesis of insulin from natural and synthetic A and B chains. II. Isolation of insulin from recombination mixtures of natural A and B chains. Biochemistry. 1967 Sep;6(9):2642–2654. doi: 10.1021/bi00861a002. [DOI] [PubMed] [Google Scholar]
- Katzen H. M., Tietze F. Studies on the specificity and mechanism of action of hepatic glutathione-insulin transhydrogenase. J Biol Chem. 1966 Aug 10;241(15):3561–3570. [PubMed] [Google Scholar]
- Moody A. J., Stan M. A., Stan M., Gliemann J. A simple free fat cell bioassay for insulin. Horm Metab Res. 1974 Jan;6(1):12–16. doi: 10.1055/s-0028-1093895. [DOI] [PubMed] [Google Scholar]
- Mori K. F., Wood R. J. A radioreceptor assay method for insulin. J Biol Stand. 1984 Oct;12(4):427–434. doi: 10.1016/s0092-1157(84)80066-9. [DOI] [PubMed] [Google Scholar]
- Qian Y. Q., Tsou C. L. Resynthesis of insulin from its A and B chains in the presence of denaturants. Biochem Biophys Res Commun. 1987 Jul 31;146(2):437–442. doi: 10.1016/0006-291x(87)90548-1. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L. The spontaneous reoxidation of reduced beef and rat proinsulins. Proc Natl Acad Sci U S A. 1968 Jun;60(2):622–629. doi: 10.1073/pnas.60.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. G., Wang C. C., Tsou C. L. Formation of native insulin from the scrambled molecule by protein disulphide-isomerase. Biochem J. 1988 Oct 15;255(2):451–455. doi: 10.1042/bj2550451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varandani P. T., Nafz M. A. Enzymatic destruction of immunoreactivity in proinsulin and insulin and activation of their scrambled forms. Arch Biochem Biophys. 1970 Dec;141(2):533–537. doi: 10.1016/0003-9861(70)90171-2. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Tsou C. L. Interaction and reconstitution of carboxyl-terminal-shortened B chains with the intact A chain of insulin. Biochemistry. 1986 Sep 9;25(18):5336–5340. doi: 10.1021/bi00366a052. [DOI] [PubMed] [Google Scholar]
- Wang Z. X., Ju M., Tsou C. L. Number of ways of joining SH groups to form multi-peptide chain proteins. J Theor Biol. 1987 Feb 7;124(3):293–301. doi: 10.1016/s0022-5193(87)80117-0. [DOI] [PubMed] [Google Scholar]
- Zahn H., Gutte B., Pfeiffer E. F., Ammon J. Resynthese von Insulin aus präoxydierter A-Kette und reduzierter B-Kette. Justus Liebigs Ann Chem. 1966 Feb;691:225–231. doi: 10.1002/jlac.19666910132. [DOI] [PubMed] [Google Scholar]


