Abstract
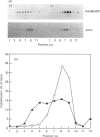
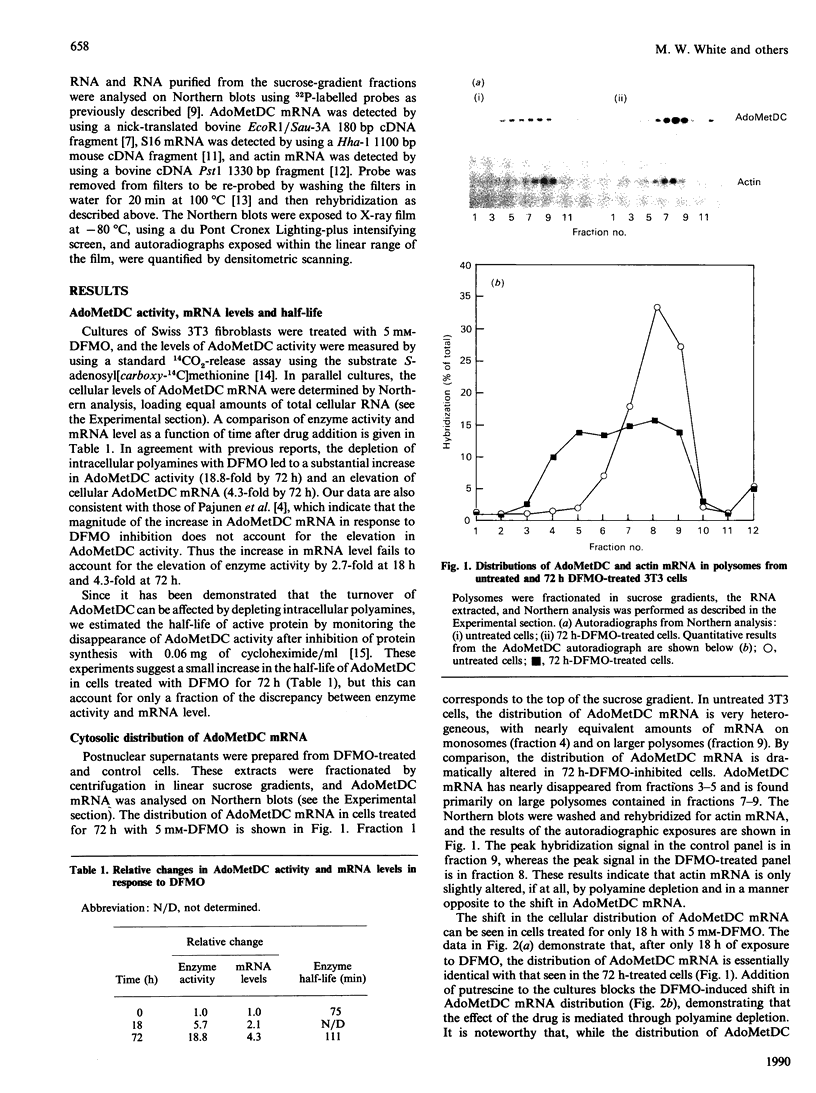
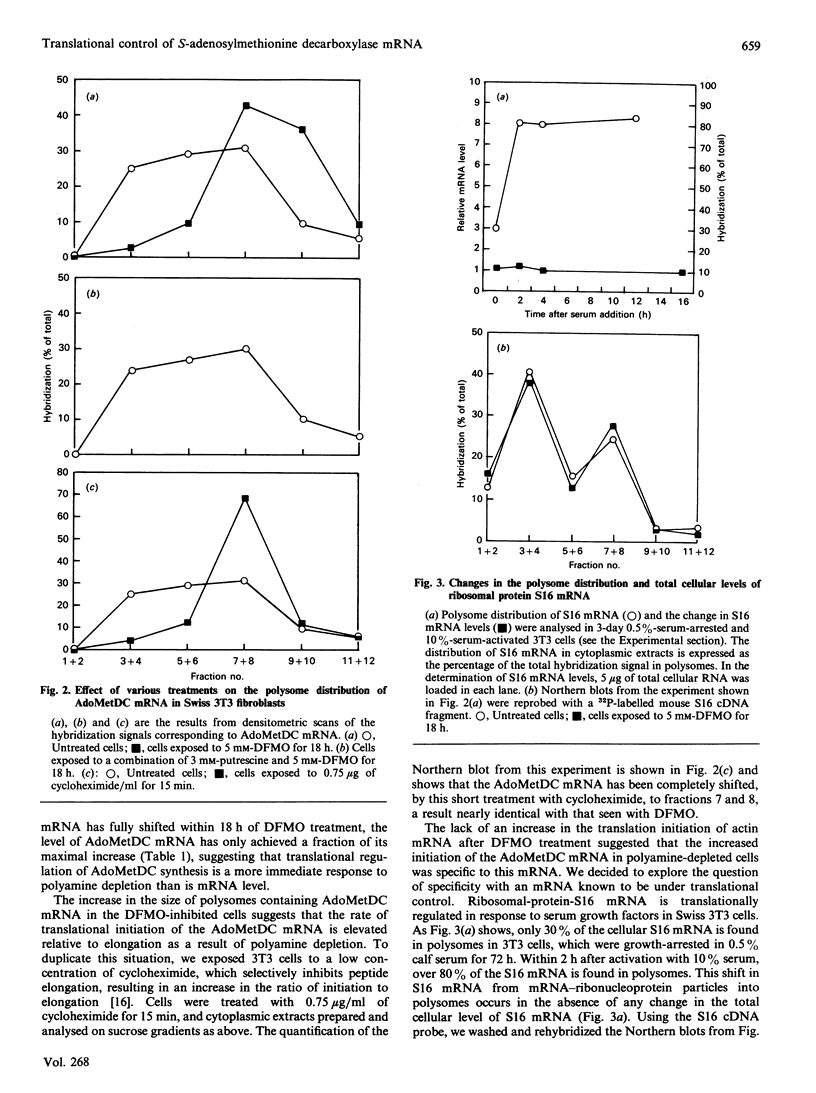
S-Adenosylmethionine decarboxylase (AdoMetDC) activity was elevated 18.8-fold in Swiss 3T3 fibroblasts which were depleted of cellular polyamines by using the inhibitor difluoromethylornithine (DFMO). Although the cellular level of AdoMetDC mRNA and the half-life of active AdoMetDC protein were also increased (4.3- and 1.5-fold respectively), together they could not account for the magnitude of the increase in AdoMetDC activity. These data suggested that the translation of AdoMetDC mRNA must be increased in the polyamine-depleted cells to account fully for the elevation in activity. The cellular distribution of AdoMetDC mRNA was examined in the polyamine-depleted cells, and it was found almost exclusively associated with large polysomes. In contrast, AdoMetDC mRNA in untreated controls was very heterogeneous, with the proportion associated with monosomes equal to that associated with large polysomes. The shift of the AdoMetDC message into large polysomes occurred within 18 h after addition of DFMO to the cultures and could be reversed by adding exogenous putrescine. The effect of polyamine depletion on AdoMetDC translation was specific, since there was no change in the distribution in polysomes of either actin mRNA or the translationally controlled mRNA encoding ribosomal protein S16 in the DFMO-inhibited cells. Thus the translational efficiency of AdoMetDC mRNA in vivo is regulated either directly or indirectly by the concentration of intracellular polyamines through a mechanism involving translational initiation, which results in a change in the number of ribosomes associated with this mRNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bethell D. R., Hibasami H., Pegg A. E. Regulation of polyamine content in cultured fibroblasts. Am J Physiol. 1982 Nov;243(5):C262–C269. doi: 10.1152/ajpcell.1982.243.5.C262. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. Polyamine accumulation during lymphocyte transformation and its relation to the synthesis, processing, and accumulation of ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4479–4487. doi: 10.1021/bi00746a028. [DOI] [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Inhibition of translation of mRNAs for ornithine decarboxylase and S-adenosylmethionine decarboxylase by polyamines. J Biol Chem. 1987 Feb 25;262(6):2427–2430. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Mach M., White M. W., Neubauer M., Degen J. L., Morris D. R. Isolation of a cDNA clone encoding S-adenosylmethionine decarboxylase. Expression of the gene in mitogen-activated lymphocytes. J Biol Chem. 1986 Sep 5;261(25):11697–11703. [PubMed] [Google Scholar]
- Pajunen A., Crozat A., Jänne O. A., Ihalainen R., Laitinen P. H., Stanley B., Madhubala R., Pegg A. E. Structure and regulation of mammalian S-adenosylmethionine decarboxylase. J Biol Chem. 1988 Nov 15;263(32):17040–17049. [PubMed] [Google Scholar]
- Pegg A. E. Investigation of the turnover of rat liver S-adenosylmethionine decarboxylase using a specific antibody. J Biol Chem. 1979 May 10;254(9):3249–3253. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Increased content of mRNA for a precursor of S-adenosylmethionine decarboxylase in rat prostate after treatment with 2-difluoromethylornithine. J Biol Chem. 1986 Oct 15;261(29):13833–13837. [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Regulation of S-adenosylmethionine decarboxylase activity in rat liver and prostate. J Biol Chem. 1985 Aug 15;260(17):9583–9588. [PubMed] [Google Scholar]
- Stimac E., Morris D. R. Messenger RNAs coding for enzymes of polyamine biosynthesis are induced during the G0-G1 transition but not during traverse of the normal G1 phase. J Cell Physiol. 1987 Dec;133(3):590–594. doi: 10.1002/jcp.1041330323. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Wagner M., Perry R. P. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol Cell Biol. 1985 Dec;5(12):3560–3576. doi: 10.1128/mcb.5.12.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. W., Kameji T., Pegg A. E., Morris D. R. Increased efficiency of translation of ornithine decarboxylase mRNA in mitogen-activated lymphocytes. Eur J Biochem. 1987 Dec 30;170(1-2):87–92. doi: 10.1111/j.1432-1033.1987.tb13670.x. [DOI] [PubMed] [Google Scholar]