Abstract
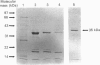
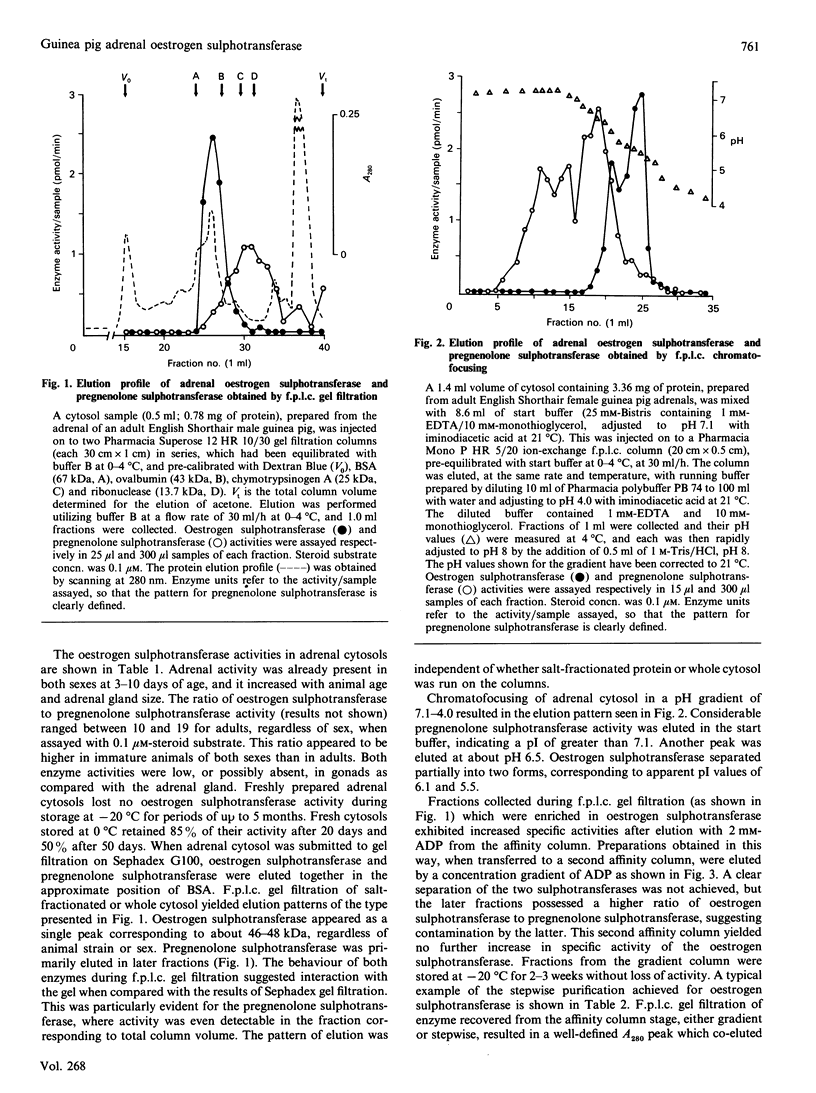
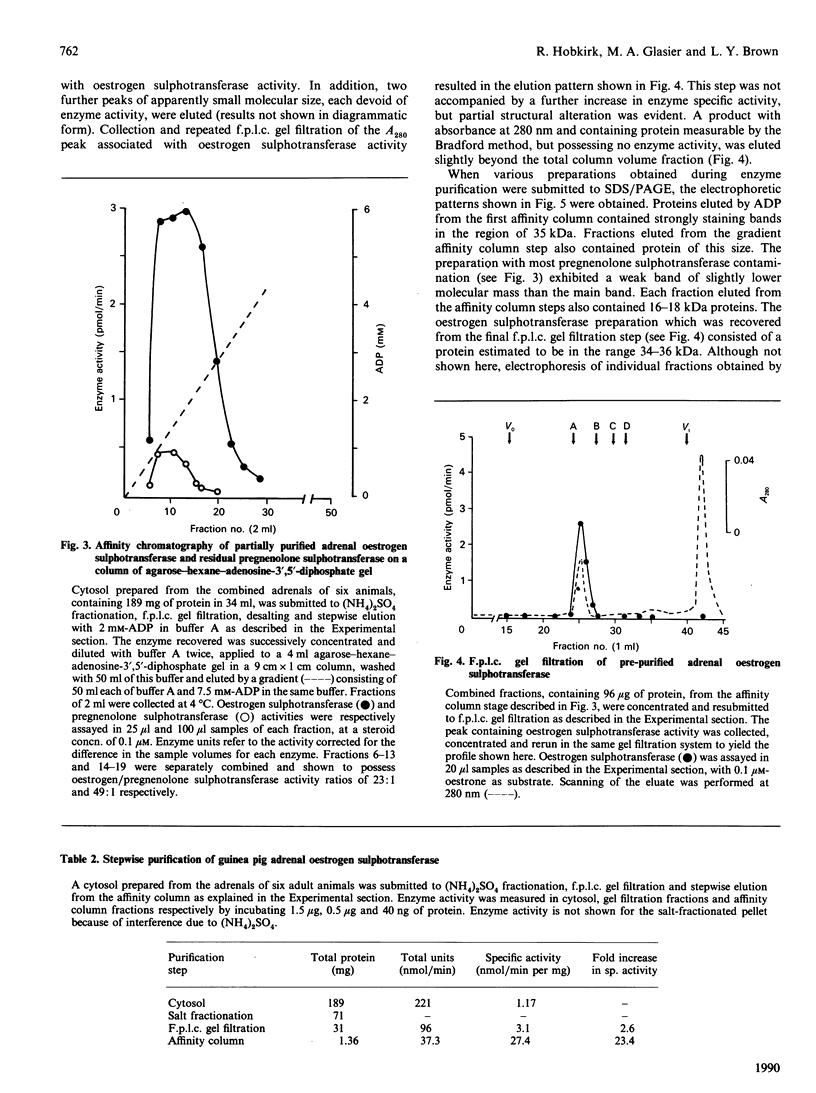
An oestrogen sulphotransferase, active towards both oestrone and oestradiol, and of high specific activity, is present in cytosol prepared from adrenal glands of both sexes of English Shorthair and Hartley guinea pigs. The ovarian and testicular cytosolic activities of this enzyme are markedly low in comparison with the adrenal activity. The adrenal enzyme is distinct from an accompanying pregnenolone sulphotransferase as judged by f.p.l.c. gel filtration, chromatofocusing, and differences in activation brought about by the addition of thiol groups. The oestrogen sulphotransferase behaved as a 67 kDa protein on a Sephadex G100 column and as a 48 kDa protein on f.p.l.c. gel-filtration columns. Two forms of the enzyme with apparent pI values of 6.1 and 5.5 were eluted during f.p.l.c. chromatofocusing. Sequential salt fractionation, f.p.l.c. gel filtration and elution from an agarose-hexane-adenosine-3',5'-diphosphate affinity gel has resulted in a preparation which, when resubmitted to f.p.l.c. gel filtration, yields a considerably purified oestrogen sulphotransferase. When submitted to SDS/polyacrylamide-gel electrophoresis under reducing conditions, a main protein band of 34-36 kDa is observed. It is suggested that the enzyme may exist as a dimer in the cytosol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. B., Ellyard R. K., Low J. Enzymic synthesis of steroid sulphates. IX. Physical and chemical properties of purified oestrogen sulphotransferase from bovine adrenal glands, the nature of its isoenzymic forms and a proposed model to explain its wave-like kinetics. Biochim Biophys Acta. 1974 Nov 25;370(1):160–188. doi: 10.1016/0005-2744(74)90042-4. [DOI] [PubMed] [Google Scholar]
- Adams J. B., Low J. Enzymic synthesis of steroid sulphates. X. Isolation of oestrogen sulphotransferase from bovine placenta and comparison of its properties with adrenal oestrogen sulphotransferase. Biochim Biophys Acta. 1974 Nov 25;370(1):189–196. doi: 10.1016/0005-2744(74)90043-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brooks S. C., Battelli M. G., Corombos J. D. Endocrine steroid sulfotransferases: porcine endometrial estrogen sulfotransferase. J Steroid Biochem. 1987 Feb;26(2):285–290. doi: 10.1016/0022-4731(87)90084-7. [DOI] [PubMed] [Google Scholar]
- Clarke C. L., Adams J. B., Wren B. G. Induction of estrogen sulfotransferase in the human endometrium by progesterone in organ culture. J Clin Endocrinol Metab. 1982 Jul;55(1):70–75. doi: 10.1210/jcem-55-1-70. [DOI] [PubMed] [Google Scholar]
- Dick C. M., Hobkirk R. Characteristics and behavior during partial purification of estrogen sulfotransferase of guinea pig liver and chorion. Biochim Biophys Acta. 1987 Sep 11;925(3):362–370. doi: 10.1016/0304-4165(87)90203-0. [DOI] [PubMed] [Google Scholar]
- Dick C. M., Hobkirk R. Estrogen sulfotransferase of mouse placenta: behaviour and characteristics during partial purification. Biochem Cell Biol. 1987 Oct;65(10):847–852. doi: 10.1139/o87-110. [DOI] [PubMed] [Google Scholar]
- Freeman D. J., Saidi F., Hobkirk R. Estrogen sulfotransferase activity in guinea pig uterus and chorion. J Steroid Biochem. 1983 Jan;18(1):23–27. doi: 10.1016/0022-4731(83)90325-4. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Cardy C. A., Saidi F., Kennedy T. G., Girard L. R. Development and characteristics of an oestrogen sulphotransferase in placenta and uterus of the pregnant mouse. Comparison between mouse and rat. Biochem J. 1983 Nov 15;216(2):451–457. doi: 10.1042/bj2160451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobkirk R., Cardy C. UDPGA-dependent estrogen glucuronyltransferase of guinea-pig uterus: assay, temporal relationships in pregnancy and some characteristics. J Steroid Biochem. 1980 Sep;13(9):1039–1045. doi: 10.1016/0022-4731(80)90135-1. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Girard L. R., Durham N. J., Khalil M. W. Behavior of mouse placental and uterine estrogen sulfotransferase during chromatography and other procedures. Biochim Biophys Acta. 1985 Apr 5;828(2):123–129. doi: 10.1016/0167-4838(85)90047-0. [DOI] [PubMed] [Google Scholar]
- Hobkirk R. Heterogeneity of guinea pig chorion and liver estrogen sulfotransferases. J Steroid Biochem. 1988 Jan;29(1):87–91. doi: 10.1016/0022-4731(88)90380-9. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Musey P., Nilsen M. Chromatographic separation of estrone and 17 beta-estradiol conjugates on DEAE-Sephadex. Steroids. 1969 Aug;14(2):191–206. doi: 10.1016/0039-128x(69)90033-6. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Nilsen M., Blahey P. R. Conjugation of urinary phenolic steroids in the nonpregnant human female with particular reference to estrone sulfate. J Clin Endocrinol Metab. 1969 Mar;29(3):328–337. doi: 10.1210/jcem-29-3-328. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Renaud R., Raeside J. I. Partial characterization of steroid sulfohydrolase and steroid sulfotransferase activities in purified porcine Leydig cells. J Steroid Biochem. 1989 Mar;32(3):387–392. doi: 10.1016/0022-4731(89)90211-2. [DOI] [PubMed] [Google Scholar]
- Hobkirk R. Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles. Can J Biochem Cell Biol. 1985 Nov;63(11):1127–1144. doi: 10.1139/o85-141. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyers S. A., Lozon M. M., Corombos J. D., Saunders D. E., Hunter K., Christensen C., Brooks S. C. Induction of porcine uterine estrogen sulfotransferase activity by progesterone. Biol Reprod. 1983 Jun;28(5):1119–1128. doi: 10.1095/biolreprod28.5.1119. [DOI] [PubMed] [Google Scholar]
- Moore S. S., Thompson E. O., Nash A. R. Oestrogen sulfotransferase: isolation of a high specific activity species from bovine placenta. Aust J Biol Sci. 1988;41(3):333–341. doi: 10.1071/bi9880333. [DOI] [PubMed] [Google Scholar]
- Nash A. R., Glenn W. K., Moore S. S., Kerr J., Thompson A. R., Thompson E. O. Oestrogen sulfotransferase: molecular cloning and sequencing of cDNA for the bovine placental enzyme. Aust J Biol Sci. 1988;41(4):507–516. doi: 10.1071/bi9880507. [DOI] [PubMed] [Google Scholar]
- Rozhin J., Huo A., Zemlicka J., Brooks S. C. Studies on bovine adrenal estrogen sulfotransferase. Inhibition and possible involvement of adenine-estrogen stacking. J Biol Chem. 1977 Oct 25;252(20):7214–7220. [PubMed] [Google Scholar]
- Saunders D. E., Lozon M. M., Corombos J. D., Brooks S. C. Role of porcine endometrial estrogen sulfotransferase in progesterone mediated downregulation of estrogen receptor. J Steroid Biochem. 1989 Jun;32(6):749–757. doi: 10.1016/0022-4731(89)90450-0. [DOI] [PubMed] [Google Scholar]
- Singer S. S. Enzymatic sulfation of steroids. VI. A simple, rapid method for routine enzymatic preparation of 3'-phosphoadenosine-5'-phosphosulfate. Anal Biochem. 1979 Jul 1;96(1):34–38. doi: 10.1016/0003-2697(79)90550-5. [DOI] [PubMed] [Google Scholar]
- Strott C. A., Goff A. K., Lyons C. D. Purification of a pregnenolone-binding protein in the soluble fraction of the guinea-pig adrenal cortex: differentiation from pregnenolone sulfotransferase. J Steroid Biochem. 1983 Apr;18(4):489–498. doi: 10.1016/0022-4731(83)90070-5. [DOI] [PubMed] [Google Scholar]