Abstract
Objective:
To determine the utility of Autologous Skin Cell Suspension (ASCS) in closing full-thickness (FT) defects from injury and infection.
Background:
Although ASCS has documented success in closing partial-thickness burns, far less is known about the efficacy of ASCS in FT defects.
Methods:
Fifty consecutive patients with FT defects (burn 17, necrotizing infection 13, crush 7, degloving 5, and other 8) underwent closure with the bilayer technique of 3:1 widely meshed, thin, split-thickness skin graft and 80:1 expanded ASCS. End points were limb salvage rate, donor site reduction, operative and hospital throughput, incidence of complications, and re-epithelialization by 4, 8, and 12 weeks.
Results:
Definitive wound closure was achieved in 76%, 94%, and 98% of patients, at 4, 8, and 12 weeks, respectively. Limb salvage occurred in 42/43 patients (10 upper and 33 lower extremities). The mean area grafted was 435 cm2; donor site size was 212 cm2, representing a potential reduction of 50%. The mean surgical time was 71 minutes; the total operating room time was 124 minutes. The mean length of stay was 26.4 days; the time from grafting to discharge was 11.2 days. Four out of 50 patients (8%) required 6 reoperations for bleeding (1), breakdown (4), and amputation (1). Four out of 50 patients (8%) developed hypertrophic scarring, which responded to silicone sheeting (2) and laser resurfacing (2). The mean follow-up was 92.7 days.
Conclusions:
When used for the closure of FT wounds, point-of-care ASCS is effective and safe. Benefits include rapid re-epithelialization, high rate of limb salvage, reduction of donor site size and morbidity, and low incidence of hypertrophic scarring.
Keywords: burn injury, burn care, wound care, autologous skin cell suspension, skin graft
Due in part to constraints imposed during the COVID era, breakthroughs have emerged that have changed how we treat patients with deep partial-thickness (PT) and full-thickness (FT) wounds.1 Resuscitation practices have become more conservative, in terms of fluid requirements, as colloids and pressors help titrate goal-directed therapy.2 Noninvasive, point-of-care ultrasound is emerging as an important adjunct to monitor and optimize oxygen delivery and end-organ perfusion. Lessons learned from the care of COVID patients have improved pulmonary, cardiac, and renal critical care.3–5 Furthermore, antibiotic stewardship and infection control continue to decrease the incidence of hospital-acquired infections.6–9 Nutrition and metabolic support have become increasingly important to optimize and even accelerate wound healing.10,11 Given the limited access to the operating theater during the early COVID era, even the timing and methods of wound excision have been reassessed.1,12,13 Finally, options for temporary and permanent wound closure have increased exponentially, with the commercialization of new synthetic and biologic skin substitutes.14
Cellular therapy for wound closure has possibly reached a tipping point, in which both large and small defects can be closed more efficiently and more robustly, and certainly with smaller donor sites, than previous approaches. Recent innovations are quickly progressing from experimental to adjunctive to definitive methods of wound closure,15,16 sometimes at a fraction of the cost of other alternatives. Autologous Skin Cell Suspension (ASCS), or “spray-on skin,” has documented success in closing PT burns,17–20 but far less is known about the efficacy of ASCS in FT injuries. Three recent multi-institutional, randomized, controlled trials support the efficacy of ASCS compared to standard of care, with the major benefit of donor site reduction.21–23 However, questions remain about optimal wound bed preparation, timing of closure, impact on operative and hospital throughput, and cost-effectiveness of this new technology. The purpose of this project was to determine the utility of ASCS in closing FT defects, from a variety of thermal and nonthermal causes.
METHODS
Study Design
We performed a retrospective, descriptive review of our first 50 consecutive patients who underwent autologous skin suspension for the closure of FT wounds and defects. These patients were treated at a single center, using the bilayer technique of point-of-care ASCS (which includes keratinocytes, fibroblasts, and melanocytes) sprayed over 3:1 widely meshed, split-thickness skin graft (STSG). This operative procedure was introduced at the same time as the creation of a new service line for hospital-based plastic and reconstructive surgery. This research was approved by our institutional review board (WakeMed IRB# 28) and followed all Strengthening the Reporting of Observational studies in Epidemiology guidelines for cohort studies.https://www.strobe-statement.org (see supplemental digital material, Table 1, for Strengthening the Reporting of Observational studies in Epidemiology checklist, Supplemental Digital Content 1, http://links.lww.com/SLA/F152). All researchers completed CITI training.
TABLE 1.
Patient Characteristics
| Total N=50 | Nonburn N=33 | Burn N=17 | P | |
|---|---|---|---|---|
| Age (Med, IQR) | 48 (31, 67) | 55 (44, 69) | 33 (26, 41) | 0.0001 |
| BMI (Med, IQR) | 26.2 (23.9, 33.2) | 26.5 (23.9, 33.2) | 25.8 (23.3, 32.9) | 0.62 |
| Sex, N (%) | ||||
| Female | 20 (40.0) | 15 (45.5) | 5 (29.4) | 0.27 |
| Male | 30 (60.0) | 18 (54.6) | 12 (70.6) | — |
| Race, N (%) | — | — | — | 0.12 |
| Asian | 2 (4.1) | 2 (6.1) | 0 | — |
| Black | 17 (34.7) | 8 (24.2) | 9 (52.9) | — |
| Hispanic | 3 (6.1) | 1 (3.0) | 2 (11.8) | — |
| Other | 2 (4.1) | 2 (6.1) | 0 | — |
| White | 26 (51.0) | 20 (60.6) | 6 (35.3) | — |
| Comorbidities, N (%) | ||||
| Diabetes | 7 (14.0) | 6 (18.2) | 1 (5.9) | 0.26 |
| Malnutrition | 5 (10.0) | 3 (9.1) | 2 (11.8) | 0.77 |
| Heart | 18 (36.7) | 16 (48.5) | 2 (12.5) | 0.01 |
| Liver | 2 (4.0) | 2 (6.1) | 0 | 0.30 |
| Lungs | 9 (18.0) | 8 (24.2) | 1 (5.9) | 0.11 |
| Kidneys | 9 (18.0) | 9 (27.3) | 0 | 0.02 |
| Substance use | 5 (10.0) | 2 (6.1) | 3 (17.7) | 0.20 |
| Any | 37 (64.0) | 30 (90.9) | 7 (41.2) | <0.01 |
| Smoking, N (%) | ||||
| Former | 12 (24.0) | 8 (24.2) | 4 (23.5) | 0.47 |
| Current | 10 (20.0) | 5 (15.2) | 5 (29.4) | |
| ASA, N (%) | — | — | — | 0.01 |
| 1 | 1 (1.9) | 0 | 1 (5.9) | — |
| 2 | 11 (22.6) | 3 (11.1) | 8 (47.1) | — |
| 3 | 25 (47.2) | 19 (52.8) | 6 (35.3) | — |
| 4 | 13 (28.3) | 11 (36.1) | 2 (11.8) | — |
BMI indicates body mass index.
Patient Population and Setting
From August 2022 through November 2023, 50 adults and children with full-thickness wounds and defects throughout the body due to diverse causes were managed by a single surgical team at an urban, level 1 trauma center. WakeMed Health and Hospitals is a not-for-profit, independent, community health care system with 4 hospitals, 970 licensed in-patient beds, and over 300K ED visits per year. The Raleigh campus serves as a teaching facility for residents, medical students, and physician assistant students from both Campbell University and the University of North Carolina at Chapel Hill. The Department of Plastic and Reconstructive Surgery belongs to an integrated group practice of over 800 physicians and advanced practice providers.
Main Outcome Measures
The primary end point of wound closure was defined as nearly complete (>98%) re-epithelialization, assessed at 4, 8, and 12 weeks after ASCS. Additional outcomes included size of donor site reduction and limb salvage rate. Secondary outcome measures included incidence of complications (both within and after 30 d), need for reoperation, incidence of hypertrophic scarring at both the donor site and reconstructed area, development of neuropathic pain, and additional reconstructive procedures, such as laser resurfacing, nerve release, and fat grafting. For operative and hospital throughput, we examined operative times (in-room to incision, in-room to out-of-room, and incision to closure), plus the total length of stay and number of days from the index procedure to discharge. For financial considerations, we looked at total hospital charges, hospital charges over the duration of admission, and physician charges for the index case.
Data Collection and Analysis
Patients were prospectively entered into a registry for performance and quality improvement. After obtaining IRB approval, we then performed a retrospective data extraction from the EPIC electronic health record (EHR), which included clinical, photographic, throughput, and financial information. To minimize bias, the authors used only objective data obtained from the EHR. In addition to descriptive statistics, we compared burn to nonburn patients, using two-tailed, nonpaired Student t test with unequal variance for continuous data and χ2 analysis for categorical data. P values <0.05 were assigned statistical significance.
Surgical Technique
Wound Bed Preparation
FT wounds and mixed FT/PT wounds throughout the body underwent excisional preparation with a combination of scalpel, curette, scissors, and Versajet (Smith + Nephew, London, UK), removing necrotic and infected debris, back to healthy appearing, viable tissue. Hemostasis was achieved with a combination of direct pressure and epinephrine/thrombin solution, with only minimal use of electrocautery. Infected, contaminated, or questionably viable wounds were temporarily closed with xenograft, allograft, or synthetic skin substitute. Clean, mature wounds with good vascularity underwent one-stage, definitive closure with STSG and ASCS.
Calculation of STSG and ASCS Needed for Wound Closure
The total surface area of the wound was measured, and the STSG needed for closure (anticipating a 2:1 expansion from 3:1 meshing) was calculated by dividing this area by a factor of 2. The total volume of ASCS needed, based on an 80:1 expansion, to treat both the primary wound and the STSG donor site was determined by combining the surface area of both wounds and dividing this by a factor of 80. Each square centimeter of skin harvested for ASCS was used to cover 80 cm2 of wound and reconstituted in 1 mL of buffer. As an example, a 500 cm2 wound would require a 250 cm2 donor site, to provide a functional 2-fold STSG expansion, plus a 9.375 cm2 donor site, to provide an 80:1 ASCS expansion, used to spray over the primary wound and secondary STSG donor site (which combined would total 750 cm2 + 9.375 cm2).
Donor Skin Harvesting
STSG was harvested at 0.012 to 0.014-inch thickness, and the ASCS donor was harvested at 0.006 to 0.008-inch thickness, with an air-powered Zimmer dermatome (Zimmer Biomet, Warsaw, IN). STSG was meshed at 3:1 ratio.
ASCS Preparation
The ReCell Autologous Skin Harvesting Device was used to prepare ASCS (Avita Medical, Valencia, CA). Trypsin was activated and heated for 10 minutes, followed by a 10-minute incubation with the thin biopsy specimen. After deactivation of the trypsin with a buffer soak, the epidermis was then scraped off of the dermis, and epidermal fragments were mechanically disaggregated with a scalpel. These fragments were reconstituted in an additional buffer, and this solution was passed through a microscopic filter to create a single-cell suspension. Previously published work indicates that ASCS contains 1.7×106 cells/mL, with 75.5% viability by trypan blue staining. The cell population, as determined by flow cytometry, includes keratinocytes (64.3%), fibroblasts (30.3%), and melanocytes (3.5%).24
ASCS Grafting
After placing the expanded STSG over the wound and securing the graft with staples or sutures, fibrin sealant (Tisseel, Baxter, Deerfield, IL) was sprayed over the interstices and used as a tissue glue for the ASCS, which was aerosolized and delivered onto the graft. ASCS was then covered with a nonadherent, small pore, low-absorbency primary dressing (Telfa Clear Wound Dressing, Cardinal Health, Dublin, OH), followed by a secondary antimicrobial 3% bismuth dressing (Xeroform Occlusive Petrolatum Gauze, Covidian, Dublin, IR), and a tertiary compressive dressing to mechanically secure the construct, such as a crepe bandage, elastic wrap, or negative pressure wound therapy (Wound VAC, 3M, Maplewood, MN).
Postoperative Care
Dressings were kept in place until postoperative days 3 to 5, at which point the secondary and tertiary dressings were removed and replaced. The primary dressing was kept intact until postoperative days 6 to 8. Wound care was transitioned to a nonadherent oil-emulsion layer (Adaptic, 3M, Maplewood, MN) plus bacitracin, covered with a compressive absorbent layer that was changed daily until the keratinocyte-STSG construct was stable and dry (indicative of functional keratin production). Regarding systemic antibiotics, patients continued treatment with their pregrafting regimen or were started on prophylactic antibiotics, which were discontinued at the time of their first dressing change. Topical silver antibiotics were not used, due to potential keratinocyte cytotoxicity. Activity restrictions were determined on a case-by-case basis in collaboration with occupational and physical therapy. Management of edema remained a priority not only in the immediate postoperative period but for weeks to months after wound closure.
Wound Biopsies
After accruing 50 patients in our cohort study, we obtained 3 mm punch biopsies on an additional patient at 1, 2, and 6 weeks postoperatively to assess and document the evolving architecture of the epidermal–dermal interface. The patient, who provided written consent, had sustained a right leg degloving injury after a fall, and he underwent 3:1 STSG and ASCS, for a 252 cm2 full-thickness defect. In clinic, after anesthetizing the area with 1% lidocaine with epinephrine injected deep into the wound, we sampled a representative area that included both STSG and ASCS only with a 3-mm punch biopsy probe. Specimens were fixed in formalin, sectioned with a microtome, stained with hematoxylin and eosin, and reviewed with a dermatopathologist.
RESULTS
Patient Demographics
From August 2022 through November 2023, 50 patients with FT wounds from diverse etiologies underwent excision and closure with 3:1 meshed STSG and 80:1 ASCS, across 53 sessions. The mean age was 48.7, with a range of 1 to 94 years. The population included 30 males and 20 females. Distribution of race was White (26), Black (17), Hispanic (3), Asian (2), and other (2). Risk factors for wound healing or significant past medical history included obesity (BMI>30) (16), cardiovascular disease (16), active smoking (10), pulmonary disease (9), renal dysfunction (9), substance abuse (8), diabetes (8), malnutrition (6), and hepatic dysfunction (4); 37 patients had at least one risk factor or medical illness. The distribution of ASA score was ASA 1 (1), ASA 2 (11), ASA 3 (25), and ASA 4 (13) (Table 1).
Wound Characteristics
Etiology of the wounds included burns (17), necrotizing soft tissue infection (12), crush injury (7), open abdomen (5), degloving (5), skin necrosis from intravenous drug use (1), keloid resection (1), and ulcer from peripheral vascular disease (2). Seventy-six percent of patients had FT-only defects, and 24% had mixed FT and deep PT defects. Location of wounds included lower extremities (33), abdomen (11), upper extremities (10), and chest (5), with 9 patients having multiple locations. The mean MESS (Mangled Extremity Severity Score) for our 30 trauma patients with extremity injuries was 3.87, with a range of 1 to 8 and an SD of 1.96. Seven patients had MESS scores of 6 or greater. See Table 2 (53 wounds in 50 patients).
TABLE 2.
Wound Characteristics
| Total N=53 | >Nonburn N=36 | Burn N=17 | P | |
|---|---|---|---|---|
| Wound depth, N (%) | — | — | — | <0.001 |
| Full-thickness | 41 (77.4) | 34 (94.4) | 7 (41.2) | — |
| Partial and full thickness | 12 (22.6) | 2 (5.6) | 10 (58.8) | — |
| MESS grade (30 trauma patients) | N=30 | N=13 | N=17 | — |
| Mean | 3.87 | 4.85 | 3.11 | 0.01 |
| SD | 1.96 | 1.68 | 1.87 | — |
| 1 (no. patients, %) | 4 (13.3) | 0 (0) | 4 (23.5) | — |
| 2 | 4 (13.3) | 0 (0) | 4 (23.5) | — |
| 3 | 6 (20) | 4 (30.8) | 2 (11.8) | — |
| 4 | 5 (16.7) | 2 (15.4) | 3 (17.6) | — |
| 5 | 4 (13.3) | 2 (15.4) | 2 (11.8) | — |
| 6 | 4 (13.3) | 3 (23.1) | 1 (5.9) | — |
| 7 | 2 (6.7) | 1 (7.7) | 1 (5.9) | — |
| 8 | 1 (3.3) | 1(7.7) | 0 (0) | — |
| Wound preparation, N (%) | 0.07 | |||
| Allograft | 11 (20.8) | 6 (16.7) | 5 (29.4) | — |
| Integra | 3 (5.6) | 3 (8.3) | 0 | — |
| Kerecis | 26 (49.1) | 21 (58.3) | 5 (29.4) | 0.05 |
| Myriad | 1 (1.9) | 1 (2.8) | 0 | — |
| None | 12 (22.6) | 5 (13.9) | 7 (41.2) | — |
| Wound size, cm2 (Med, IQR) | 350 (154, 610) | 400 (149, 691) | 296 (158, 420) | 0.12 |
| Donor size, cm2 (Med, IQR) | 175 (77, 300) | 180 (79, 350) | 120 (70, 210) | 0.10 |
| Recell size, cm2 (Med, IQR) | 498 (220, 915) | 590 (236, 993) | 436 (213, 630) | 0.10 |
| Donor site coverage, n (%) | 0.70 | |||
| Kerecis | 29 (54.7) | 21 (58.3) | 8 (47.1) | |
| Suprathel | 17 (32.1) | 11 (30.6) | 6 (35.3) | |
| Xeroform | 7 (13.2) | 4 (11.1) | 3 (17.7) | |
| EBL, mL (Med, IQR) | 50 (25, 100) | 50 (23, 75) | 50 (30, 100) | 0.42 |
EBL indicates estimated blood loss.
Characteristics of Treated Areas
Twelve patients (24%) had excision with immediate grafting, but 38 patients (76%) had staged excision before definitive closure. Temporary graft material included xenograft in 27 patients, allograft in 8 patients, and synthetic skin substitute in 3 patients. Patients with nonburn wounds were more likely to be treated with piscine xenograft than patients with burn injury. The mean surface area of the wounds grafted was 435 cm2 (range 30–1608 cm2). The mean area of the donor site was 212 cm2 (15–804 cm2). This represents a 50% reduction in donor site if compared with grafts normally meshed at 1.5:1 (which functionally effects a 1:1 expansion) and a 25% reduction if compared with grafts meshed at 2:1 (which yields a 1.5:1 expansion). Mean size of ASCS application was 636 cm2 (45–2212 cm2). See Table 2 (53 wounds in 50 patients).
Healing Outcomes
Definitive wound closure was achieved in 76%, 94%, and 98% of patients, at 4, 8, and 12 weeks, respectively. Limb salvage occurred in 42/43 patients (10 upper and 33 lower extremities). One patient, who sustained a crush injury from a forklift, with a MESS of 7, required below-knee amputation several months later for intractable distal lymphedema and recurrent cellulitis. Four patients had delayed healing of donor sites beyond 4 weeks, with three patients receiving additional biologic grafts (1 xenograft, 2 allograft) to facilitate wound closure. Two patients developed pseudomonas soft tissue infections that responded to topical and systemic antibiotics. One patient required repeat STSG for partial graft loss at the recipient site, due to mechanical shearing. One patient with closed wounds at 8 weeks had donor and recipient site breakdown by 12 weeks, due to Munchausen’s syndrome.
Complications and Adverse Events
Four patients (8%) required 6 reoperation for bleeding (1), breakdown (4), and amputation (1). One patient with a previous open abdomen developed a transient enterocutaneous fistula that healed spontaneously with bowel rest. In terms of late sequelae, only four patients (8%) developed hypertrophic scarring, which responded to laser resurfacing (2) or compression and silicone sheeting (2); 2 patients developed hypertrophic scars at their donor sites, which were successfully managed with lasers. One patient underwent successful nerve decompression and fat grafting for refractory neuropathic pain. No patients developed scar contractures, banding, or excessive tightness. Overall complications and adverse events occurred in 12 patients or 24% of the cohort.
Comparison of Burn With Nonburn Patients
Compared with nonburn patients, burn patients, on average, were younger (33.1 vs 56.7 y), had a lower ASA class (2.5 vs 3.2), had a lower MESS score (3.1 vs 4.8), and had a shorter length of stay (13.6 vs 32.5 d) (all P values < 0.05), due in part to a shorter time from ASCS to discharge (6.8 vs 13.2 d) (P=0.055). Wound size, donor site reduction, estimated blood loss, case time, operating room (OR) time, wound closure rates, complication rates, and length of follow-up were similar between burn and nonburn patients. See Tables 1, 2, and 3 [data reported as medians, with interquartile range (IQRs)].
TABLE 3.
Throughput and Financial Analysis
| Total | Nonburn | Burn | P | |
|---|---|---|---|---|
| Incision time, min (Med, IQR) | 64 (52, 82) | 66 (54, 88) | 58 (52, 76) | 0.65 |
| OR time, min (Med, IQR) | 116 (101, 140) | 115 (101, 139) | 112 (100, 136) | 0.70 |
| Incision/OR time ratio (Med, IQR) | 0.55 (0.48, 0.61) | 0.57 (0.50, 0.62) | 0.52 (0.48, 0.58) | 0.55 |
| Total length of stay, days (Med, IQR) | 21 (12, 35) | 26 (19, 37) | 12 (6, 21) | 0.01 |
| Post-ASCS LOS, days (Med, IQR) | 7 (4, 14) | 10 (5, 14) | 5 (4, 7) | 0.09 |
| Total charges for hospitalization (Med, IQR) | $336, 176 ($185,391, $524,962) |
$469,131 ($310,715, $685,409) |
$185,391 ($116,719, $276915) |
0.0001 |
| Physician charges for index case (Med, IQR) | $23,190 ($18,317, $43,325) |
$23,852 ($18,956, $51,155) |
$20,960 ($13,816, $25,759) |
0.43 |
| Follow-up, days (Med, IQR) | 59 (35, 101) | 61 (40, 135) | 49 (23, 88) | 0.26 |
Case Studies
Case 1
This patient was a 57-year-old man with Fournier’s gangrene who underwent radical excision of his scrotum, penile shaft skin, and lower abdominal skin and fascia. Following transposition of the testes to a subcutaneous pocket in the thighs, he had staged closure of his defect with allograft, piscine xenograft, and finally, ASCS sprayed over 3:1 meshed STSG. His penis was reconstructed with sheet grafts and circumcision. His diverting colostomy was taken down 3 months later, and he reports the ability to have an erection with penetrative intercourse. See Supplemental Digital Figure 1, Supplemental Digital Content 2, http://links.lww.com/SLA/F153.
Case 2
This patient was a 49-year-old man who developed necrotizing fasciitis of his chest and left upper extremity, after wrestling with his son, who had just tested positive for a group A streptococcal infection of his oropharynx. After radical debridement, he underwent staged closure with piscine xenograft, bilaminate synthetic skin substitute, and eventually ASCS sprayed over 3:1 meshed STSG. Negative pressure wound therapy was used at most stages to secure his grafts. See Supplemental Digital Figure 2, Supplemental Digital Content 2, http://links.lww.com/SLA/F153.
Case 3
This patient was a 66-year-old woman who sustained a severe crush injury to her right leg, after being pinned between 2 golf carts, resulting in a tibial fracture and loss of soft tissue. She underwent staged closure with a xenograft and ultimately ASCS sprayed over a 3:1 meshed STSG. Although she developed early hypertrophic scarring of her recipient and donor site, she responded well to 3 sessions of pulsed dye laser photothermolysis and fractional CO2 laser ablation. She also underwent open superficial peroneal nerve decompression and percutaneous sural nerve release with fat grafting, for focal neuropathic pain. See Supplemental Digital Figure 3, Supplemental Digital Content 2, http://links.lww.com/SLA/F153.
Biopsy Data
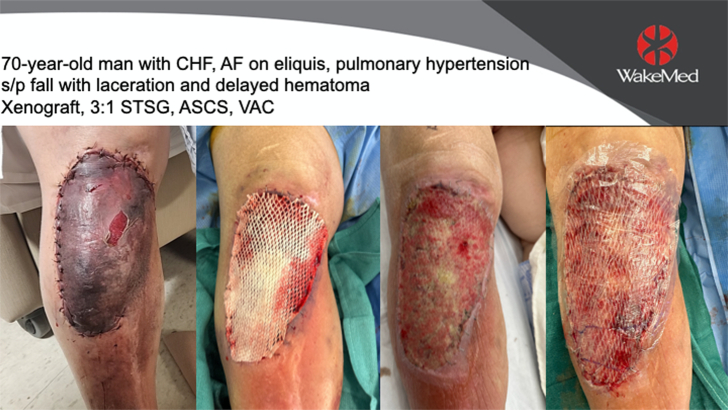
The patient selected for sequential punch biopsies was a 70-year-old man with congestive heart failure with atrial fibrillation and pulmonary hypertension, on apixaban, who fell and sustained a laceration with hematoma, resulting in a full-thickness defect over his leg and knee. He underwent staged closure with a piscine xenograft and negative pressure wound therapy, followed 1 week later by 3:1 STSG and 80:1 ASCS (Fig. 1). One week after closure, a fragile neoepidermis was observed, 6 to 8 keratinocytes thick, over disorganized granulation tissue (Fig. 2). By 2 weeks, biopsies demonstrate early lining up of the stratum basale and stratum corneum, with elements of the strata spinosum and granulosum emerging (Fig. 3). At 6 weeks after grafting, mature keratin sheets are forming on the outer epidermis, along with rete ridges and a clear basement membrane. Deep to an organized layer of basal keratinocytes, a pseudo-papillary dermis has emerged, which includes a myxoid extracelluar matrix and maturing collagen bundles, all devoid of sweat glands and hair follicles (Fig. 4).
FIGURE 1.
70-year-old man with congestive heart failure, atrial fibrillation, and pulmonary hypertension, on apixiban, who fell and sustained a laceration, delayed hematoma, and full-thickness tissue loss. He underwent excision and coverage with a piscine xenograft, followed 1 week later by STSG and ASCS, with negative pressure wound therapy.
FIGURE 2.
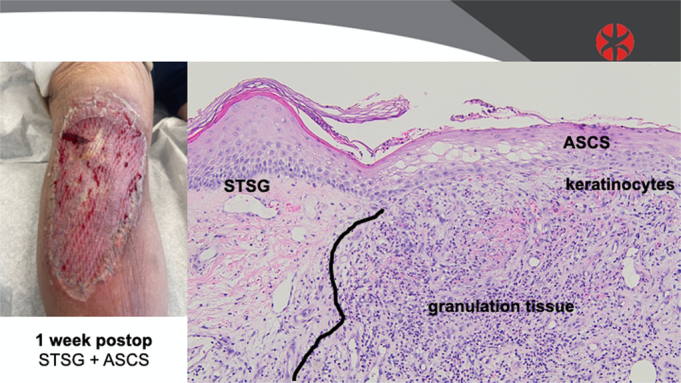
Biopsy data: 1 week after grafting with ASCS and 3:1 meshed STSG.
FIGURE 3.
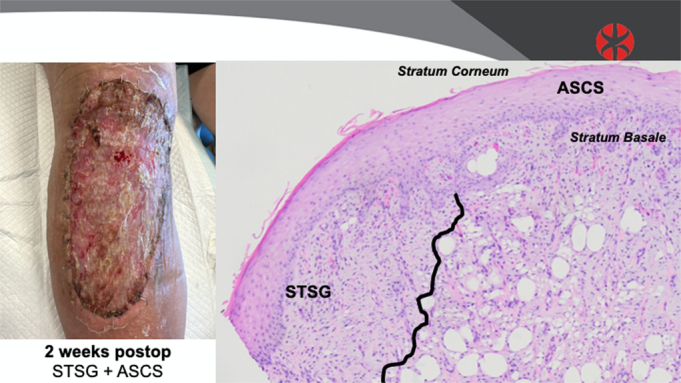
Biopsy data: 2 weeks after grafting with ASCS and 3:1 meshed STSG.
FIGURE 4.
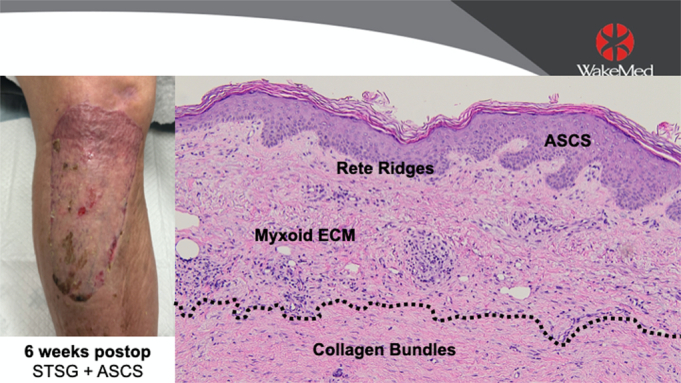
Biopsy data: 6 weeks after grafting with ASCS and 3:1 meshed STSG.
Financial Analysis
The median total hospital charge for all patients was $336,176 (IQR $185,391–$524,962), while the median physician charge for the index case was $23,190 (IQR $18,317–$43,325). Median total hospital charge was significantly higher for nonburn patients ($469,131) than burn patients ($185,391) (P<0.001), although median physician charge was similar for both groups ($23,852 vs $20,960) (NS) (Table 3).
Throughput Analysis
Mean surgical time, from “incision to close,” was 71 minutes (range 35–173 min). Mean total operative time, from “in-room to out-of-room” was 124 minutes (range 71–231 min). The mean time from in-room to incision was 41 minutes (range 19–83 min). Surgical and operative times tended to improve over the course of the study but did not reach statistical significance, due in part to the high variability observed throughout the period (Supplemental Fig. 4, Supplemental Digital Content 2, http://links.lww.com/SLA/F153). Mean length of stay was 26.4 days (range 0–129 d). Time from ASCS grafting to discharge was 11.2 days (0–67 d). Four patients underwent STSG and ASCS as an out-patient (Table 3).
DISCUSSION
In this retrospective review of our first 50 patients with FT wounds, treated at an urban, level 1 trauma center with “spray-on skin,” we provide compelling data that support the use of 80:1 expanded ASCS sprayed over 3:1 meshed STSG, for the definitive closure of these defects. Wound closure was achieved in 76%, 94%, and 98% of patients, at 4, 8, and 12 weeks after grafting, respectively. Limb salvage occurred in 42/43 patients. The mean area grafted was 435 cm2, while the donor site size was 212 cm2. This represents a potential 50%–25% reduction in skin graft requirements, depending on STSG mesh ratios used without ASCS. The mean surgical time was 71 minutes; total OR time was 124 minutes. The mean length of stay was 26.4 days; the time from grafting to discharge was 11.2 days. In terms of complications, 4/50 patients (8%) required reoperation for bleeding (1), breakdown (2), and amputation (1). Four out of 50 patients (8%) developed hypertrophic scarring with neuropathic pain, which responded to laser resurfacing and fat grafting.
Compared with nonburn patients, burn patients were younger (33.1 vs 56.7 y), had a lower ASA class (2.5 vs 3.2), had a lower MESS score (3.1 vs 4.8), and had a shorter length of stay (13.6 vs 32.5 d), due in part to a shorter time from ASCS to discharge (6.8 vs 13.2 d) (all P values <0.05). Wound size, donor site reduction, estimated blood loss, case time, OR time, wound closure rates, complication rates, and length of follow-up were similar between burn and nonburn patients. Total hospital charges for burn patients were significantly lower than nonburn patients ($185,391 vs $469,131, P<0.001), whereas physician surgical charges were similar ($20,960 vs $23,852, NS).
Since Rheinwald and Green first described the ability to culture keratinocytes in 1975,25 and Cuono reported using cultured epidermal autografts (CEAs) in pediatric burn patients in 1986,26 cellular therapy has been pursued as both a primary and secondary method of wound closure for PT and FT defects27—with mixed success. Munster documented that the use of CEAs was associated with improved mortality in patients with large burns,28 but CEAs still have considerable limitations, including the 2 to 3 week period needed to grow confluent sheets, the intensive nursing care required to protect the grafts (requiring a 1:1 nursing ratio), the long-term fragility of the grafts (which delay and limit both occupational and physical therapy), and the considerable cost, which remains around $10,000 for every 1% TBSA covered (or $50/cm2).29 We also demonstrated that CEAs contain persistent, foreign antigens that can elicit an inflammatory, second-set response.30 However, CEAs save lives and remain an integral part of the wound closure algorithm for burn surgeons, especially for injuries >50% TBSA.
In 2007 and 2012, Wood et al31,32 demonstrated that an aerosolized, skin cell suspension of keratinocytes, melanocytes, and fibroblasts could be used to achieve durable wound closure. Advantages of this innovative technique included point of care preparation in the OR, short learning curve for the surgical team, in vivo confluence of the grafted keratinocytes, simplified dressing and wound care, early patient mobilization, and significantly decreased cost ($7500 per 1920 cm2 application, or $3.9/cm2). Multiple refinements and applications have been reported in abundant cases series since then,33–47 including the use of ASCS in pediatric patients, the utility of ASCS in wounds other than burns, the efficacy of ASCS in FT defects, and success of ASCS when used with other technologies, such as tissue glue and negative pressure wound therapy.
Over the past 5 years, 3 robust randomized controlled trials have been published, supporting the use of ASCS for wound closure.21–23 In burn patients with deep PT injuries, Holmes provided evidence that ASCS induces stable short-term and long-term healing, with significantly reduced donor site size and pain, plus improved appearance, compared with STSG alone. The mean ASCS donor site was ~40 times smaller than that of the STSG, and donor sites treated with ASCS healed faster than those treated with conventional dressings. Combining ASCS with widely meshed STSG as a bilayer construct, Holmes also demonstrated, in patients with mixed depth burns without contiguous dermis, that ASCS plus expanded STSG reduced donor site requirements by 32%, and resulted in a similar safety and efficacy profile, compared with minimally expanded STSG. Eight weeks after grafting, 92% of the ASCS wounds were closed, compared to 85% of the control group.
With strong data that ASCS plus widely meshed STSG (ASCS+STSG) is not inferior to the standard of care (minimally expanded STSG) and that ASCS yields benefits in reduction of donor site size and pain, Henry recently published the first randomized control trial in patients with full-thickness defects due to surgical and traumatic wounds, but excluding burns.23 Eight weeks after grafting, complete closure was observed in 65% of the ASCS+STSG group, compared with 58% of the STSG control group (P<0.01). Furthermore, the ASCS+STSG group required 27.4% less donor skin (P<0.001). The mean area treated by ASCS+STSG was 216 cm2, compared with 212 cm2 for the STSG control group. No differences were observed between the groups, regarding patient complications, adverse device-related events, or final scarring measured by patient and observer scar assessment score.
Several logistical, operational, and financial issues are relevant. Over the course of this case series, total procedural times tended to improve, despite some variability in preincision anesthesia induction. ASCS preparation can add ~30 minutes to operative time if done sequentially, but we utilize a 2-team approach, which allows for simultaneous skin processing on the back table, with concurrent wound preparation and grafting with expanded STSG, by the primary team. The learning curves for keratinocyte harvest and processing are quite short; surgical assistants rapidly learn this skill and can be supervised by the attending surgeon. Communication at the start of the procedure is critical to establish the anticipated flow and momentum of the operation, which requires a dermatome, a mesher capable of at least 3:1 expansion, the ASCS kit, tumescence for the donor site, topical epinephrine and thrombin solution for wound hemostasis, adhesive tissue glue, skin substitutes for the donor site, and copious dressing supplies.
The relative cost of the ASCS kit is quite small ($7500), compared with total hospital charges generated for burn patients ($185,391), but especially for patients with nonburn wounds ($469,391). Because the surgical team does the actual work to harvest and prepare ASCS for grafting, the only charges incurred are the purchase of the kit and the professional fees of the surgeon, not the actual graft materials, driving down the cost of ASCS to <10% of CEAs. Furthermore, ASCS may be the critical event that drives the timing of discharge, since this occurs in the final third of hospitalization. These patients tend to have 3 cost spikes during their stay: the initial workup and treatment in the emergency department, the primary intervention (repair of fracture, debridement of necrotizing infection, stabilization of soft tissue injury, as examples), and definitive closure with ASCS and STSG.
Although the current study has limitations as a retrospective review, this cohort of 50 consecutive patients with full-thickness defects is the largest cases series of ASCS and widely meshed STSG reported by a single team, at a single institution—and at the start of a new service line. Given the previous positive experience of our providers with ASCS, at other academic medical centers (Johns Hopkins University, University of North Carolina, University of Indiana, and University of Alabama—Birmingham), we did not feel compelled to include a control group, since ASCS plus widely meshed STSG was already our preferred practice and considered by many as one of several best practices for closing deep PT, mixed-depth, and FT wounds. As such, these patients were entered into a departmental registry, for later data extraction and analysis. With a wound closure rate of 94% at 8 weeks and 98% at 12 weeks, and a donor site reduction of 50%, our results are similar to and perhaps better than those of the 3 previously published RCTs.21–23
Future research will help answer questions about whether or not ASCS induces accelerated healing and if so, what cellular and biochemical mechanisms may account for these effects. Combining real-world data with financial modeling, Carter and colleagues provide convincing evidence that ASCS reduces the length of stay by over 2 days for large (>20% TBSA) and small burns.48,49 However, the impact of ASCS on length of stay for nonburn patients is not yet clear, but given the high cost associated with these wounds, the potential for an increased return on investment may be greater than for burn patients. We also recognize the need to design and execute a prospective, blinded trial comparing the use ASCS with differing STSG mesh ratios in varying locations of the body and the need to collect both patient-reported outcome measures and objective scar assessments.
In summary, when used for closure of FT wounds, point-of-care ASCS plus widely meshed STSG is effective and safe for both burn and nonburn patients. Particular benefits include rapid re-epithelialization, high rate of limb salvage, reduction of donor site size and morbidity, and low incidence of hypertrophic scarring. In our series, ASCS was not financially nor operationally prohibitive in burn or nonburn patients and was often the catalyst for discharge in both cohorts. ASCS should be considered as a method of wound closure for most FT defects.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Diana Rhyne, MBA, for assistance with IRB submission, data extraction from EPIC, and analysis of financial data.
Footnotes
Presented at the 144th Annual Scientific Meeting of the American Surgical Association, Washington, DC, April 4 to 6, 2024.
C.S.H.: consultant for Avita Medical and Vericel Corporation, receives royalties from Walters Kluwer for UpToDate. C.A.M.: consultant for Kerecis. The remaining authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
C. Scott Hultman, Email: chultman@wakemed.org.
Ursula C. Adams, Email: ursula.adams@unchealth.unc.edu.
Corianne D. Rogers, Email: corogers@wakemed.org.
Minakshi Pillai, Email: minakshi_pillai@med.unc.edu.
Samantha T. Brown, Email: samabrown@wakemed.org.
Carrie Ann McGroarty, Email: cmcgroarty@wakemed.org.
Michelle McMoon, Email: mmcmoon@wakemed.org.
M. Georgina Uberti, Email: muberti@wakemed.org.
REFERENCES
- 1.Yoon JS, Khoo KH, Akhavan AA, et al. Changes in burn surgery operative volume and metrics due to COVID-19. J Burn Care Res. 2022;43:1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2021 American Burn Association State and Future of Burn Science Working Group . Proceedings of the 2021 American Burn Association State and Future of Burn Science Meeting. J Burn Care Res. 2022;43:1241–1259. [DOI] [PubMed] [Google Scholar]
- 3.Lagziel T, Quiroga LH, Ross E, et al. The impact of different co-morbidities on clinical outcomes and resource utilization in critically ill burn and surgical patients: a population-based analysis of social determinants of health. Burns. 2024;50:823–828. [DOI] [PubMed] [Google Scholar]
- 4.Klifto KM, Shetty PN, Slavin BR, et al. Impact of nicotine/smoking, alcohol, and illicit substance use on outcomes and complications of burn patients requiring hospital admission: systematic review and meta-analysis. Burns. 2020;46:1498–1524. [DOI] [PubMed] [Google Scholar]
- 5.Klifto KM, Quiroga L, Hultman CS. Substance use and inhalation injury in adult burn patients: retrospective study of the impact on outcomes. Burns Trauma. 2019;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagziel T, Asif M, Born L, et al. Evaluating the efficacy, safety, and tolerance of silver sulfadiazine dressings once daily versus twice daily in the treatment of burn wounds. J Burn Care Res. 2021;42:1136–1139. [DOI] [PubMed] [Google Scholar]
- 7.Rostami S, Kawaji Q, Martinez SL, et al. Focused wound care handoff improves burn center physician-nursing communication and wound care education. J Burn Care Res. 2023;44:254–256. [DOI] [PubMed] [Google Scholar]
- 8.Klifto KM, Rydz AC, Biswas S, et al. Evidence-based medicine: systemic perioperative antibiotic prophylaxis for prevention of surgical-site infections in plastic and reconstructive surgery. Plast Reconstr Surg. 2023;152:1154e–1182e. [DOI] [PubMed] [Google Scholar]
- 9.Klifto KM, Gurno CF, Seal SM, Hultman CS. Factors associated with mortality following burns complicated by necrotizing skin and soft tissue infections: a systematic review and meta-analysis of individual participant data. J Burn Care Res. 2022;43:163–188. [DOI] [PubMed] [Google Scholar]
- 10.Lagziel T, Akhavan AA, Yoon JS, et al. Carry that weight! The challenge of managing weight changes during inpatient admission for patients with burn injuries ≥20% TBSA. J Burn Care Res. 2022;43:781–786. [DOI] [PubMed] [Google Scholar]
- 11.Born LJ, Quiroga LH, Lagziel T, et al. Clinical outcomes in ‘diabese’ burn patients: a systematic review and meta-analysis. Burns. 2022;48:281–292. [DOI] [PubMed] [Google Scholar]
- 12.Slavin B, Shoucair S, Klifto K, et al. Inappropriate transfer of burn patients: a 5-year retrospective at a single center. Ann Plast Surg. 2021;86:29–34. [DOI] [PubMed] [Google Scholar]
- 13.Asif M, Chin AGM, Lagziel T, et al. The added benefit of combining laser Doppler imaging with clinical evaluation in determining the need for excision of indeterminate-depth burn wounds. Cureus. 2020;12:e8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenny EM, Lagziel T, Hultman CS, Egro FM. Skin substitutes and autograft techniques: temporary and permanent coverage solutions. Clin Plast Surg. 2024;51:241–254. [DOI] [PubMed] [Google Scholar]
- 15.Domaszewska-Szostek AP, Krzyżanowska MO, Czarnecka AM, et al. Local treatment of burns with cell-based therapies tested in clinical studies. J Clin Med. 2021;10:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh M, Nuutila K, Kruse C, et al. Challenging the conventional therapy: emerging skin graft techniques for wound healing. Plast Reconstr Surg. 2015;136:524e–530e. [DOI] [PubMed] [Google Scholar]
- 17.Navarro FA, Stoner ML, Park CS, et al. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine microwound model. J Burn Care Rehabil. 2000;21:513–518. [DOI] [PubMed] [Google Scholar]
- 18.Gravante G, Di Fede MC, Araco A, et al. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33:966–972. [DOI] [PubMed] [Google Scholar]
- 19.Sood R, Roggy DE, Zieger MJ, et al. A comparative study of spray keratinocytes and autologous meshed split-thickness skin graft in the treatment of acute burn injuries. Wounds. 2015;27:31–40. [PubMed] [Google Scholar]
- 20.Bairagi A, Griffin B, Banani T, et al. A systematic review and meta-analysis of randomized trials evaluating the efficacy of autologous skin cell suspensions for re-epithelialization of acute partial thickness burn injuries and split-thickness skin graft donor sites. Burns. 2021;47:1225–1240. [DOI] [PubMed] [Google Scholar]
- 21.Holmes JH, IV, Molnar JA, Shupp JW, et al. Demonstration of the safety and effectiveness of the RECELL® System combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries. Burns. 2019;45:772–782. [DOI] [PubMed] [Google Scholar]
- 22.Holmes Iv JH, Molnar JA, Carter JE, et al. A comparative study of the ReCell® device and autologous spit-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018;39:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry S, Mapula S, Grevious M, et al. Maximizing wound coverage in full-thickness skin defects: a randomized-controlled trial of autologous skin cell suspension and widely meshed autograft versus standard autografting. J Trauma Acute Care Surg. 2024;96:85–93. [DOI] [PubMed] [Google Scholar]
- 24.Wood FM, Giles N, Stevenson A, et al. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns. 2012;38:44–51. [DOI] [PubMed] [Google Scholar]
- 25.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. [DOI] [PubMed] [Google Scholar]
- 26.Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet. 1986;1:1123–1124. [DOI] [PubMed] [Google Scholar]
- 27.Hickerson WL, Compton C, Fletchall S, et al. Cultured epidermal autografts and allodermis combination for permanent burn wound coverage. Burns. 1994;20(Suppl 1):S52–S55; discussion S55-6. [DOI] [PubMed] [Google Scholar]
- 28.Munster AM. Cultured skin for massive burns. A prospective, controlled trial. Ann Surg. 1996;224:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rue LWIII, Cioffi WG, McManus WF, et al. Wound closure and outcome in extensively burned patients treated with cultured autologous keratinocytes. J Trauma. 1993;34:662–667. [PubMed] [Google Scholar]
- 30.Hultman CS, Brinson GM, Siltharm S, et al. Allogeneic fibroblasts used to grow cultured epidermal autografts persist in vivo and sensitize the graft recipient for accelerated second-set rejection. J Trauma. 1996;41:51–58. [DOI] [PubMed] [Google Scholar]
- 31.Wood FM, Stoner ML, Fowler BV, et al. The use of a non-cultured autologous cell suspension and Integra dermal regeneration template to repair full-thickness skin wounds in a porcine model: a one-step process. Burns. 2007;33:693–700. [DOI] [PubMed] [Google Scholar]
- 32.Wood F, Martin L, Lewis D, et al. A prospective randomised clinical pilot study to compare the effectiveness of Biobrane® synthetic wound dressing, with or without autologous cell suspension, to the local standard treatment regimen in paediatric scald injuries. Burns. 2012;38:830–839. [DOI] [PubMed] [Google Scholar]
- 33.Bairagi A, Griffin B, Tyack Z, et al. Comparative effectiveness of Biobrane®, RECELL® Autologous skin Cell suspension and Silver dressings in partial thickness paediatric burns: BRACS randomised trial protocol. Burns Trauma. 2019;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campanella SD, Rapley P, Ramelet AS. A randomised controlled pilot study comparing Mepitel(®) and SurfaSoft(®) on paediatric donor sites treated with Recell(®). Burns. 2011;37:1334–1342. [DOI] [PubMed] [Google Scholar]
- 35.Wala SJ, Patterson K, Scoville S, et al. A single institution case series of ReCell® use in treating pediatric burns. Int J Burns Trauma. 2023;13:78–88. [PMC free article] [PubMed] [Google Scholar]
- 36.Malkoc A, Wong DT. Lessons learned from two survivors of greater than 90% TBSA full-thickness burn injuries using NovoSorb Biodegradable Temporizing Matrix™ and Autologous Skin Cell Suspension, RECELL™: a case series. J Burn Care Res. 2021;42:577–585. [DOI] [PubMed] [Google Scholar]
- 37.Gilleard O, Segaren N, Healy C. Experience ofrReCell in skin cancer reconstruction. Arch Plast Surg. 2013;40:627–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manning L, Ferreira IB, Gittings P, et al. Wound healing with “spray-on” autologous skin grafting (ReCell) compared with standard care in patients with large diabetes-related foot wounds: an open-label randomised controlled trial. Int Wound J. 2022;19:470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu ZC, Chen D, Guo D, et al. Randomized clinical trial of autologous skin cell suspension combined with skin grafting for chronic wounds. Br J Surg. 2015;102:e117–e123. [DOI] [PubMed] [Google Scholar]
- 40.Collins RA, Van Spronsen NR, Couch BR, et al. Autologous cell harvesting system as adjunct for soft-tissue reconstruction of necrotizing soft tissue infection. Plast Reconstr Surg Glob Open. 2022;10:e4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunne JA, Saleh DB, Rawlins JM. Management of rhinophyma with Versajet™ and ReCell®. Br J Oral Maxillofac Surg. 2013;51:e282–e284. [DOI] [PubMed] [Google Scholar]
- 42.Grossman H, Pang A, Griswold J. Treatment of severe road Rash with ReCell® autologous skin cell suspension. J Burn Care Res. 2023;44:731–733. [DOI] [PubMed] [Google Scholar]
- 43.De Angelis B, Migner A, Lucarini L, et al. The use of a non cultured autologous cell suspension to repair chronic ulcers. Int Wound J. 2015;12:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu N, Liu R, Yu P, et al. Repigmentation of nipple-areola complex after ReCell® treatment on breast vitiligo. J Cosmet Dermatol. 2022;21:2530–2534. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q, Yu N, Liu Z, et al. The clinical efficacy of ReCell® autologous cell regeneration techniques combined with dermabrasion treatment in acne scars. Aesthetic Plast Surg. 2020;44:535–542. [DOI] [PubMed] [Google Scholar]
- 46.Johnstone P, Kwei JS, Filobbos G, et al. Successful application of keratinocyte suspension using autologous fibrin spray. Burns. 2017;43:e27–e30. [DOI] [PubMed] [Google Scholar]
- 47.Carney BC, Johnson LS, Shupp JW, et al. Initial experience combining negative pressure wound therapy with autologous skin cell suspension and meshed autografts. J Burn Care Res. 2021;42:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carson JS, Carter JE, Hickerson WL, et al. Analysis of real-world length of stay data and costs associated with use of autologous skin cell suspension for the treatment of small burns in U.S. centers. Burns. 2023;49:607–614. [DOI] [PubMed] [Google Scholar]
- 49.Carter JE, Carson JS, Hickerson WL, et al. Length of stay and costs with autologous skin cell suspension versus split-thickness skin grafts: burn care data from US centers. Adv Ther. 2022;39:5191–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]