Abstract
Small dermatan sulphate proteoglycan II from cultured human skin fibroblasts interacts with type I collagen in vitro and in vivo. When fibroblasts are maintained in a type I collagen lattice the proteoglycan remains exclusively within the lattice, and its association with fibrils can be demonstrated immunocytochemically. On the basis of [35S]sulphate incorporation, small proteoglycan II comprises about 80% of total proteoglycans secreted by cells in monolayer culture. In a collagen lattice, fibroblasts down-regulate its synthesis to the level of large chondroitin sulphate/dermatan sulphate and of heparan sulphate proteoglycans, the synthesis of which remains unaffected. Compared with the product from monolayer cultures, small proteoglycan II from collagen gels contained a longer polysaccharide chain which is characterized by a larger proportion of disulphated and a smaller proportion of monosulphated glucuronic acid-containing disaccharides. The half-life varied between 60 and 110 h. It is suggested that the compositional differences between the proteoglycan from monolayer cultures and from cells in a collagen lattice are related to the slower intracellular trafficking of the proteoglycan under the latter culture conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bell E., Ehrlich H. P., Buttle D. J., Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981 Mar 6;211(4486):1052–1054. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Gasiunas N., Schor S. L. Specific association of iduronic acid-rich dermatan sulphate with the extracellular matrix of human skin fibroblasts cultured on collagen gels. Biochem J. 1983 Oct 1;215(1):107–116. doi: 10.1042/bj2150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Glössl J., Schubert-Prinz R., Gregory J. D., Damle S. P., von Figura K., Kresse H. Receptor-mediated endocytosis of proteoglycans by human fibroblasts involves recognition of the protein core. Biochem J. 1983 Nov 1;215(2):295–301. doi: 10.1042/bj2150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve H., Cully Z., Blumberg P., Kresse H. Influence of chlorate on proteoglycan biosynthesis by cultured human fibroblasts. J Biol Chem. 1988 Sep 15;263(26):12886–12892. [PubMed] [Google Scholar]
- Hatamochi A., Aumailley M., Mauch C., Chu M. L., Timpl R., Krieg T. Regulation of collagen VI expression in fibroblasts. Effects of cell density, cell-matrix interactions, and chemical transformation. J Biol Chem. 1989 Feb 25;264(6):3494–3499. [PubMed] [Google Scholar]
- Hausser H., Hoppe W., Rauch U., Kresse H. Endocytosis of a small dermatan sulphate proteoglycan. Identification of binding proteins. Biochem J. 1989 Oct 1;263(1):137–142. doi: 10.1042/bj2630137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbom E., Heinegård D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989 Apr 25;264(12):6898–6905. [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe W., Glössl J., Kresse H. Influence of monensin on biosynthesis, processing and secretion of proteodermatan sulfate by skin fibroblasts. Eur J Biochem. 1985 Oct 1;152(1):91–97. doi: 10.1111/j.1432-1033.1985.tb09167.x. [DOI] [PubMed] [Google Scholar]
- Koob T. J., Vogel K. G. Proteoglycan synthesis in organ cultures from regions of bovine tendon subjected to different mechanical forces. Biochem J. 1987 Sep 15;246(3):589–598. doi: 10.1042/bj2460589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Wight T. N. Modulation of proteoglycan metabolism by aortic smooth muscle cells grown on collagen gels. Arteriosclerosis. 1986 Nov-Dec;6(6):638–650. doi: 10.1161/01.atv.6.6.638. [DOI] [PubMed] [Google Scholar]
- Mauch C., Hatamochi A., Scharffetter K., Krieg T. Regulation of collagen synthesis in fibroblasts within a three-dimensional collagen gel. Exp Cell Res. 1988 Oct;178(2):493–503. doi: 10.1016/0014-4827(88)90417-x. [DOI] [PubMed] [Google Scholar]
- Nusgens B., Merrill C., Lapiere C., Bell E. Collagen biosynthesis by cells in a tissue equivalent matrix in vitro. Coll Relat Res. 1984 Oct;4(5):351–363. doi: 10.1016/s0174-173x(84)80003-5. [DOI] [PubMed] [Google Scholar]
- Paye M., Nusgens B. V., Lapière C. M. Modulation of cellular biosynthetic activity in the retracting collagen lattice. Eur J Cell Biol. 1987 Dec;45(1):44–50. [PubMed] [Google Scholar]
- Rauch U., Glössl J., Kresse H. Comparison of small proteoglycans from skin fibroblasts and vascular smooth-muscle cells. Biochem J. 1986 Sep 1;238(2):465–474. doi: 10.1042/bj2380465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
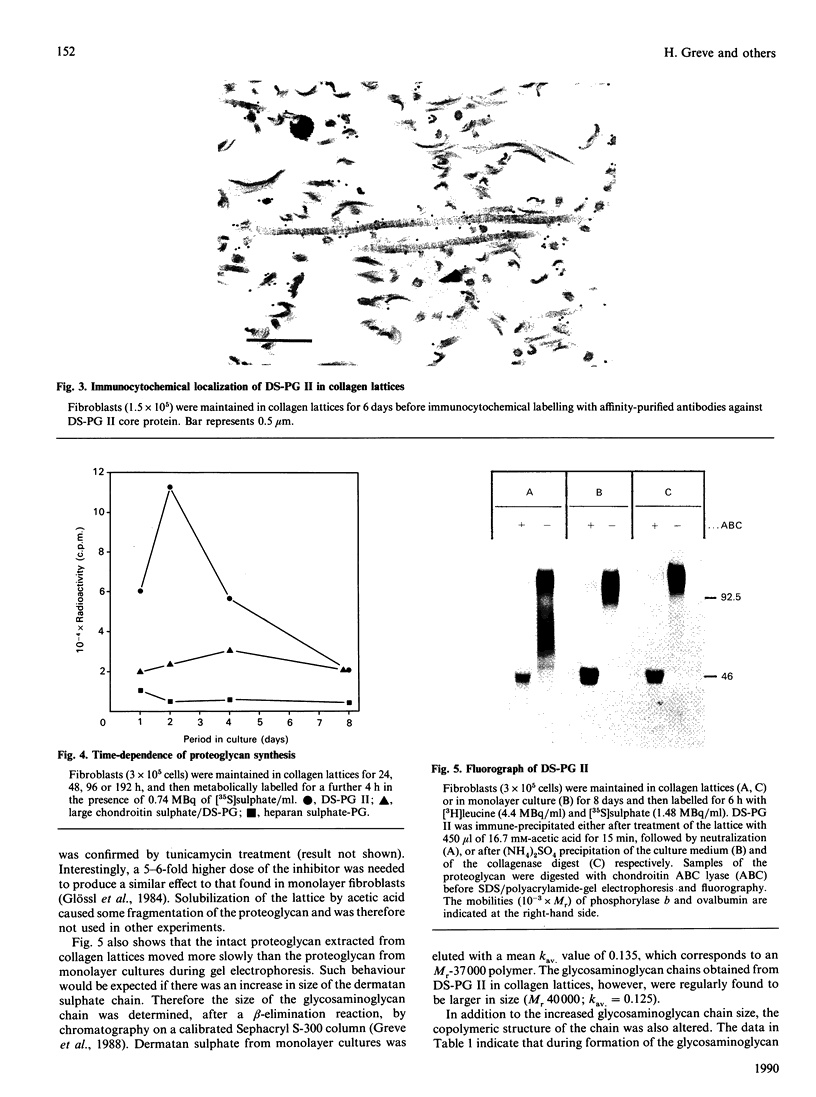
- Schlumberger W., Thie M., Rauterberg J., Kresse H., Robenek H. Deposition and ultrastructural organization of collagen and proteoglycans in the extracellular matrix of gel-cultured fibroblasts. Eur J Cell Biol. 1989 Oct;50(1):100–110. [PubMed] [Google Scholar]
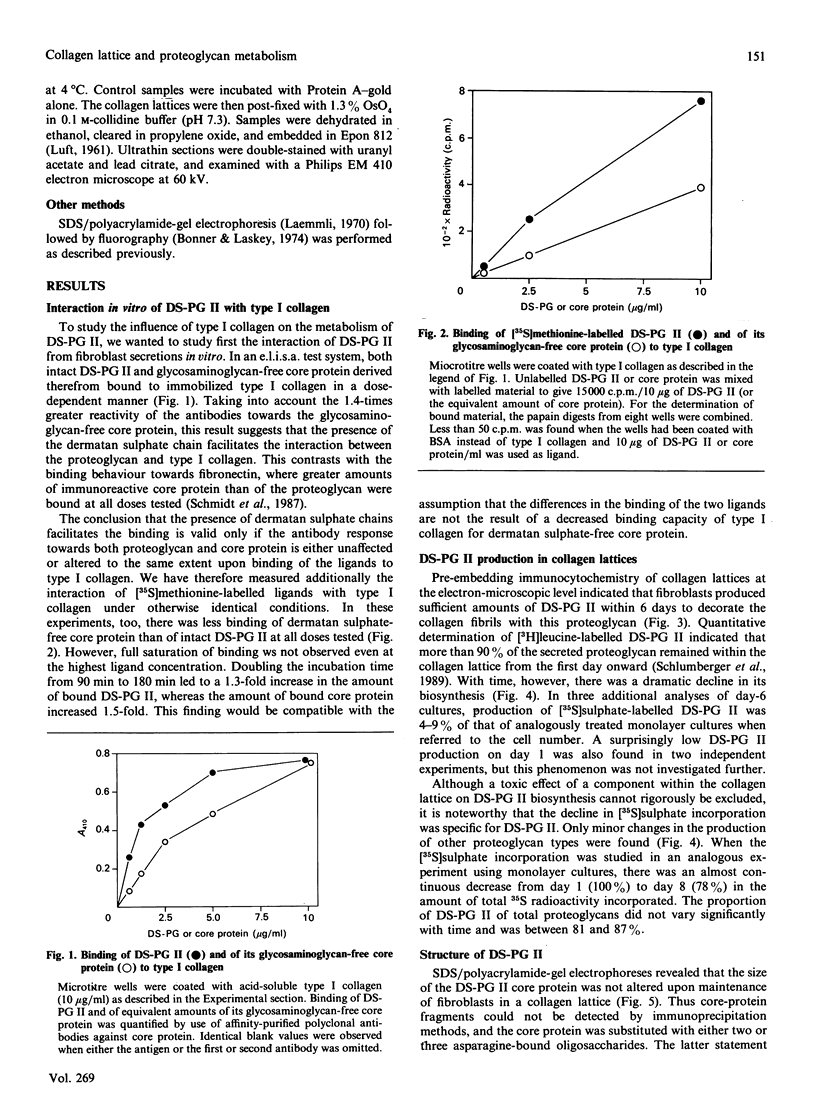
- Schmidt G., Hausser H., Kresse H. Extracellular accumulation of small dermatan sulphate proteoglycan II by interference with the secretion-recapture pathway. Biochem J. 1990 Mar 1;266(2):591–595. [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Robenek H., Harrach B., Glössl J., Nolte V., Hörmann H., Richter H., Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987 Jun;104(6):1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985 Jan;5(1):71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Hay E. D., Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, alpha-actinin, and myosin. Dev Biol. 1982 Jul;92(1):107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N., Danielsen C. C. A study of the interaction in vitro between type I collagen and a small dermatan sulphate proteoglycan. Biochem J. 1988 May 1;251(3):643–648. doi: 10.1042/bj2510643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988 Nov 17;336(6196):244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- Zebrower M. E., Kieras F. J., Brown W. T. Analysis by high-performance liquid chromatography of hyaluronic acid and chondroitin sulfates. Anal Biochem. 1986 Aug 15;157(1):93–99. doi: 10.1016/0003-2697(86)90201-0. [DOI] [PubMed] [Google Scholar]