Abstract
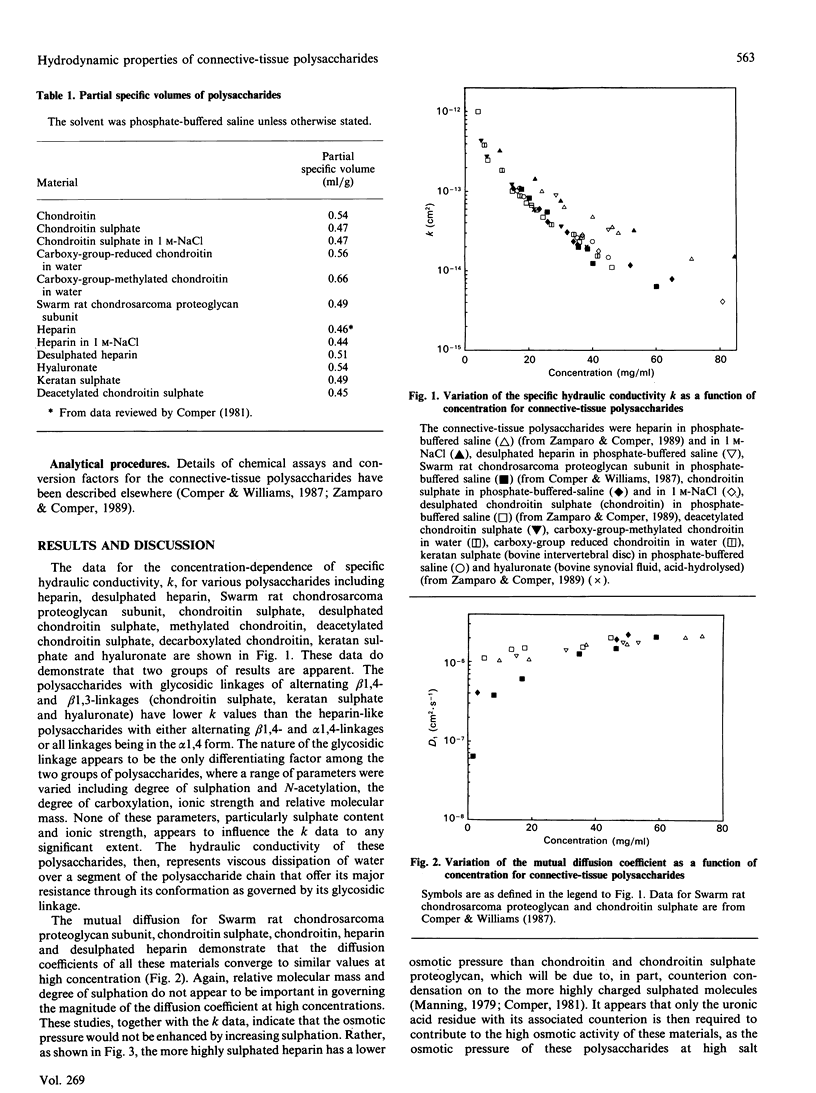
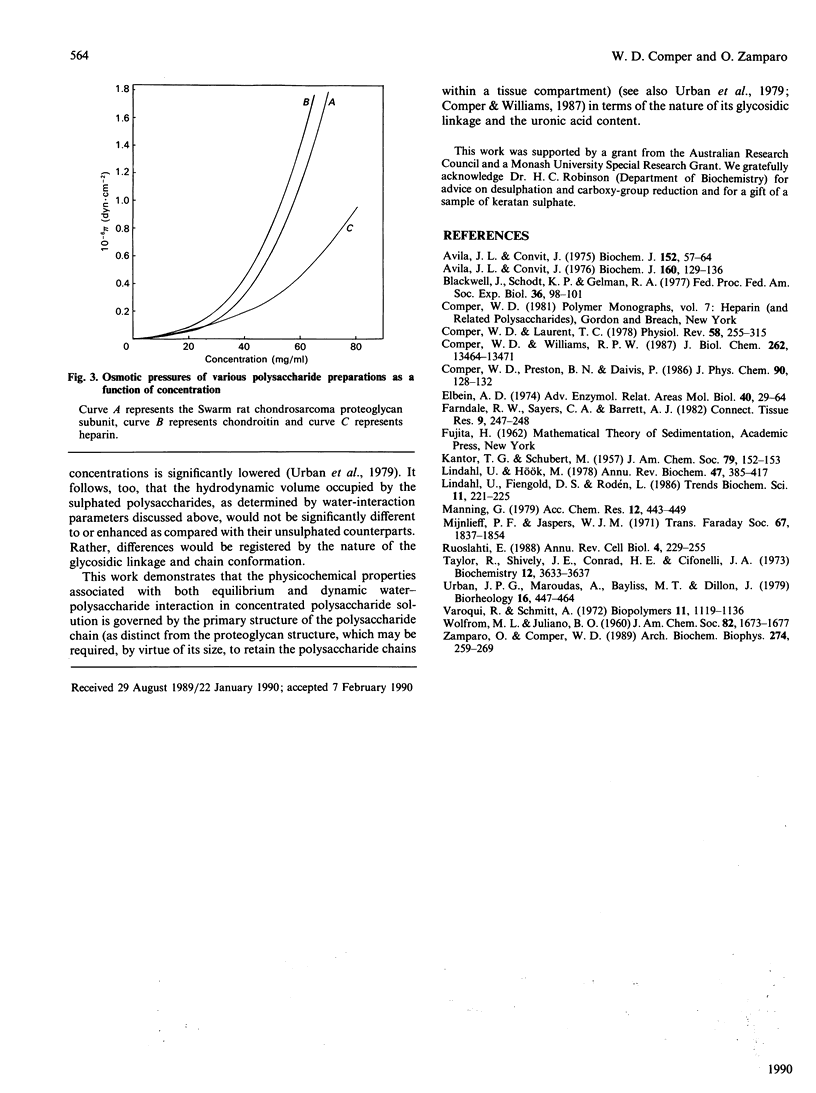
The major hydrodynamic properties of the connective-tissue polysaccharides are those that describe polysaccharide-water interaction as embodied in their osmotic-pressure and hydraulic-conductivity properties. This study shows that, for polysaccharides such as chondroitin sulphate, hyaluronate and the heparin-like polysaccharides, their hydrodynamic properties depend primarily on the presence of the uronic residue and the nature of the glycosidic linkage. Other parameters such as the degree of N-acetylation and sulphation were found not to influence these properties to any great extent. These studies particularly delineate structural-functional aspects of the connective-tissue polysaccharides in terms of their primary structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Convit J. Inhibition of leucocytic lysosomal enzymes by glycosaminoglycans in vitro. Biochem J. 1975 Oct;152(1):57–64. doi: 10.1042/bj1520057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L., Convit J. Physicochemical characteristics of the glycosaminoglycan-lysosomal enzyme interaction in vitro. A model of control of leucocytic lysosomal activity. Biochem J. 1976 Nov 15;160(2):129–136. doi: 10.1042/bj1600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J., Schodt K. P., Gelman R. A. Polysaccharide-polypeptide systems as models for heparin interactions. Fed Proc. 1977 Jan;36(1):98–101. [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Comper W. D., Williams R. P. Hydrodynamics of concentrated proteoglycan solutions. J Biol Chem. 1987 Oct 5;262(28):13464–13471. [PubMed] [Google Scholar]
- Elbein A. D. Interactions of polynucleotides and other polyelectrolytes with enzymes and other proteins. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):29–64. doi: 10.1002/9780470122853.ch2. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Shively J. E., Conrad H. E., Cifonelli J. A. Uronic acid composition of heparins and heparan sulfates. Biochemistry. 1973 Sep 11;12(19):3633–3637. doi: 10.1021/bi00743a010. [DOI] [PubMed] [Google Scholar]
- Urban J. P., Maroudas A., Bayliss M. T., Dillon J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16(6):447–464. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- Zamparo O., Comper W. D. Hydraulic conductivity of chondroitin sulfate proteoglycan solutions. Arch Biochem Biophys. 1989 Oct;274(1):259–269. doi: 10.1016/0003-9861(89)90438-4. [DOI] [PubMed] [Google Scholar]


