Abstract
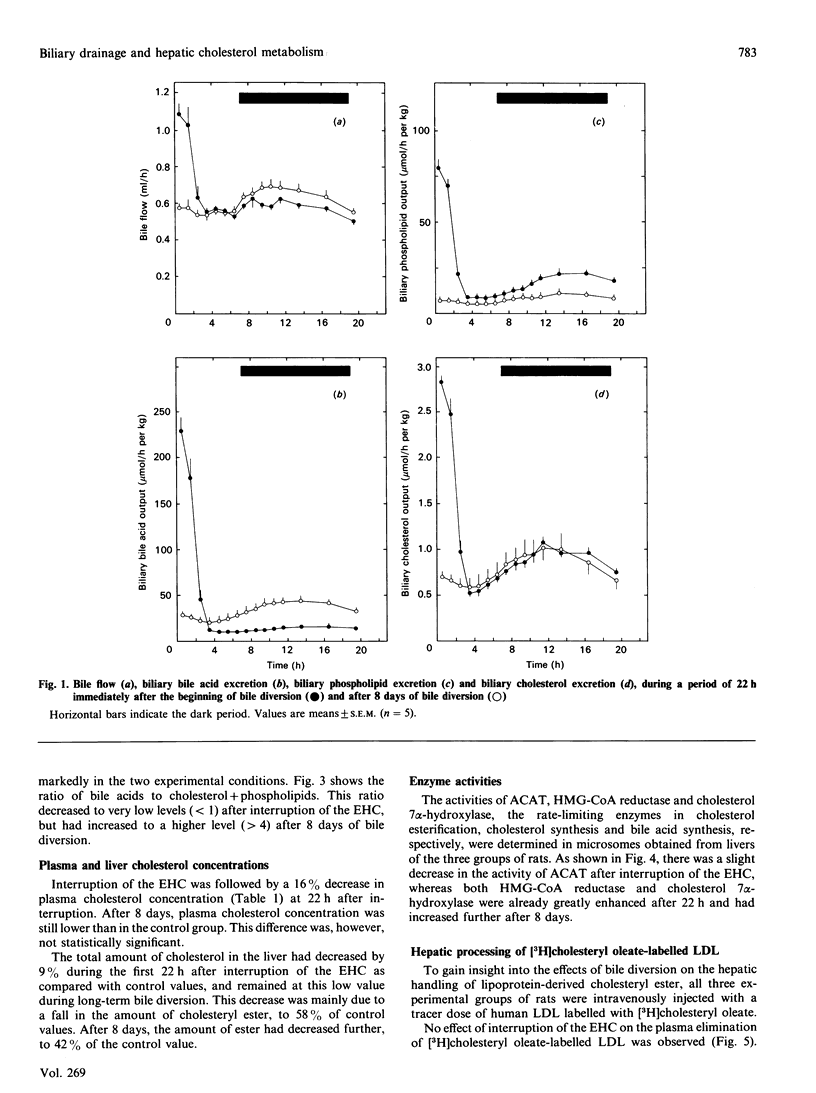
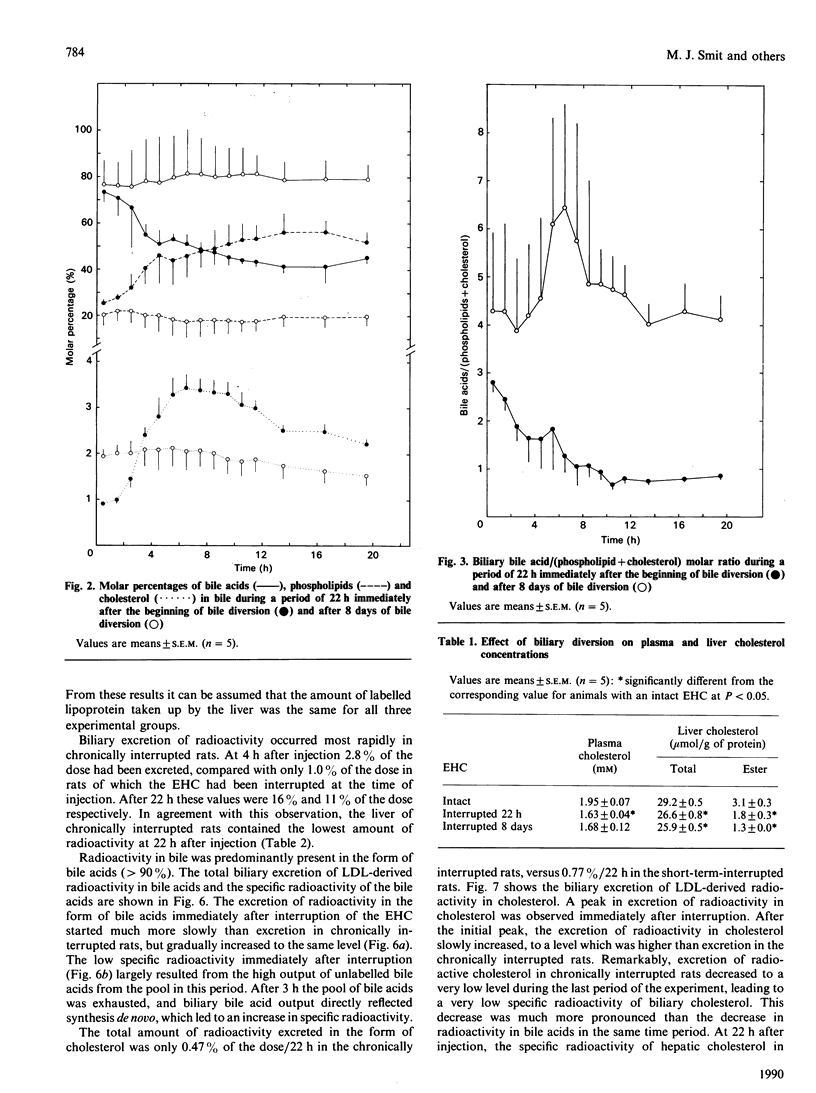
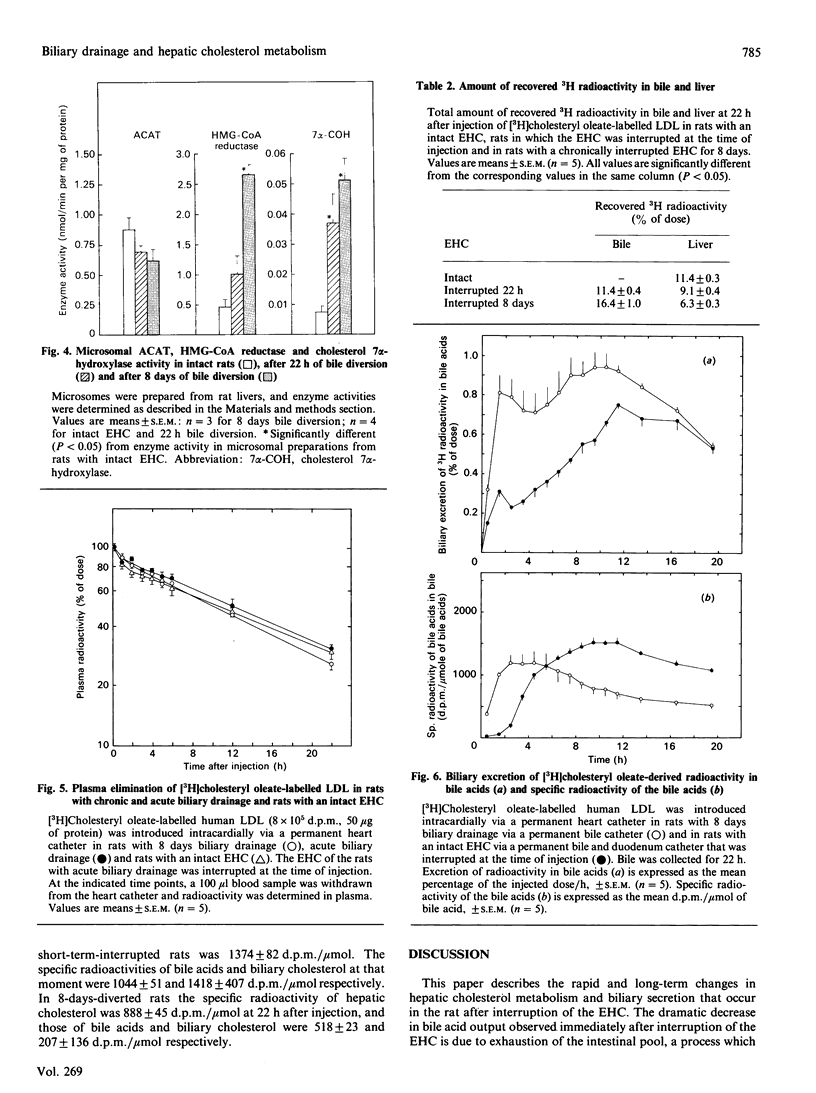
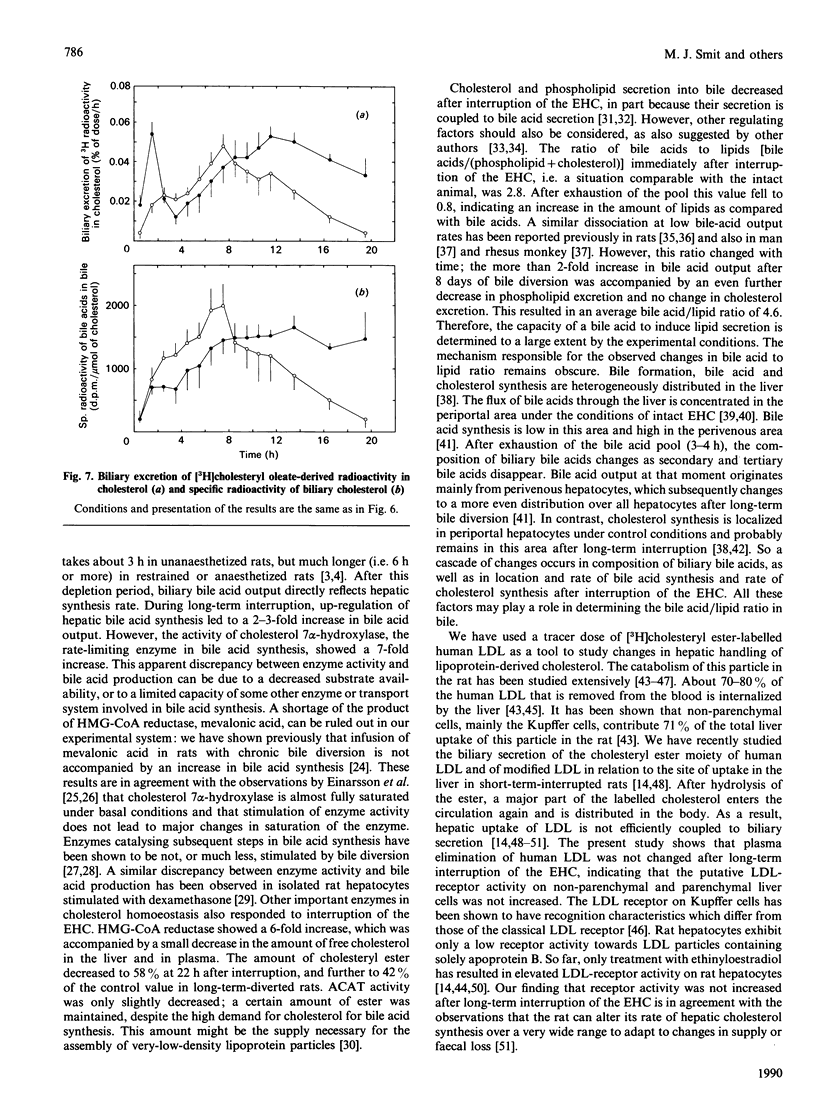
The present study concerns short- and long-term effects of interruption of the enterohepatic circulation (EHC) on hepatic cholesterol metabolism and biliary secretion in rats. For this purpose, we employed a technique that allows reversible interruption of the EHC, during normal feeding conditions, and excludes effects of anaesthesia and surgical trauma. [3H]Cholesteryl oleate-labelled human low-density lipoprotein (LDL) was injected intravenously in rats with (1) chronically (8 days) interrupted EHC, (2) interrupted EHC at the time of LDL injection and (3) intact EHC. During the first 3 h after interruption of the EHC, bile flow decreased to 50% and biliary bile acid, phospholipid and cholesterol secretion to 5%, 11% and 19% of their initial values respectively. After 8 days of bile diversion, biliary cholesterol output and bile flow were at that same level, but bile acid output was increased 2-3-fold and phospholipid output was about 2 times lower. The total amount of cholesterol in the liver decreased after interruption of the EHC, which was mainly due to a decrease in the amount of cholesteryl ester. Plasma disappearance of LDL was not affected by interruption of the EHC. Biliary secretion of LDL-derived radioactivity occurred 2-4 times faster in chronically interrupted rats as compared with the excretion immediately after interruption of the EHC. Radioactivity was mainly in the form of bile acids under both conditions. This study demonstrates the very rapid changes that occur in cholesterol metabolism and biliary lipid composition after interruption of the EHC. These changes must be taken into account in studies concerning hepatic metabolism of lipoprotein cholesterol and subsequent secretion into bile.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Balabaud C., Kron K. A., Gumucio J. J. The assessment of the bile salt-nondependent fraction of canalicular bile water in the rat. J Lab Clin Med. 1977 Feb;89(2):393–399. [PubMed] [Google Scholar]
- Bhattacharya S., Balasubramaniam S., Simons L. A. Quantification of LDL cholesteryl ester contribution to biliary steroids in the rat. Biochim Biophys Acta. 1986 May 21;876(3):413–416. doi: 10.1016/0005-2760(86)90027-5. [DOI] [PubMed] [Google Scholar]
- Billheimer J. T., Tavani D., Nes W. R. Effect of a dispersion of cholesterol in Triton WR-1339 on acyl CoA: cholesterol acyltransferase in rat liver microsomes. Anal Biochem. 1981 Mar 1;111(2):331–335. doi: 10.1016/0003-2697(81)90570-4. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Drevon C. A., Eskild W., Helgerud P., Norum K. R., Berg T. Clearance of acetyl low density lipoprotein by rat liver endothelial cells. Implications for hepatic cholesterol metabolism. J Biol Chem. 1984 Jul 25;259(14):8898–8903. [PubMed] [Google Scholar]
- Chao Y. S., Windler E. E., Chen G. C., Havel R. J. Hepatic catabolism of rat and human lipoproteins in rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11360–11366. [PubMed] [Google Scholar]
- Danielsson H., Einarsson K., Johansson G. Effect of biliary drainage on individual reactions in the conversion of cholesterol to taurochlic acid. Bile acids and steroids 180. Eur J Biochem. 1967 Jul;2(1):44–49. doi: 10.1111/j.1432-1033.1967.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M. Regulation of cholesterol metabolism in man and in other species. Klin Wochenschr. 1984 Apr 16;62(8):338–345. doi: 10.1007/BF01716251. [DOI] [PubMed] [Google Scholar]
- ERIKSSON S. Biliary excretion of bile acids and cholesterol in bile fistula rats; bile acids and steroids. Proc Soc Exp Biol Med. 1957 Mar;94(3):578–582. doi: 10.3181/00379727-94-23018. [DOI] [PubMed] [Google Scholar]
- Einarsson K., Akerlund J. E., Björkhem I. The pool of free cholesterol is not of major importance for regulation of the cholesterol 7 alpha-hydroxylase activity in rat liver microsomes. J Lipid Res. 1987 Mar;28(3):253–256. [PubMed] [Google Scholar]
- Einarsson K., Reihnér E., Björkhem I. On the saturation of the cholesterol 7 alpha-hydroxylase in human liver microsomes. J Lipid Res. 1989 Oct;30(10):1477–1481. [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Groothuis G. M., Hardonk M. J., Keulemans K. P., Nieuwenhuis P., Meijer D. K. Autoradiographic and kinetic demonstration of acinar heterogeneity of taurocholate transport. Am J Physiol. 1982 Dec;243(6):G455–G462. doi: 10.1152/ajpgi.1982.243.6.G455. [DOI] [PubMed] [Google Scholar]
- Guo L. S., Hamilton R. L., Ostwald R., Havel R. J. Secretion of nascent lipoproteins and apolipoproteins by perfused livers of normal and cholesterol-fed guinea pigs. J Lipid Res. 1982 May;23(4):543–555. [PubMed] [Google Scholar]
- Hardison W. G., Apter J. T. Micellar theory of biliary cholesterol excretion. Am J Physiol. 1972 Jan;222(1):61–67. doi: 10.1152/ajplegacy.1972.222.1.61. [DOI] [PubMed] [Google Scholar]
- Harkes L., Van Berkel J. C. Quantitative role of parenchymal and non-parenchymal liver cells in the uptake of [14C]sucrose-labelled low-density lipoprotein in vivo. Biochem J. 1984 Nov 15;224(1):21–27. doi: 10.1042/bj2240021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkes L., Van Berkel T. J. In vivo characteristics of a specific recognition site for LDL on non-parenchymal rat liver cells which differs from the 17 alpha-ethinyl estradiol-induced LDL receptor on parenchymal liver cells. Biochim Biophys Acta. 1984 Jul 6;794(2):340–347. doi: 10.1016/0005-2760(84)90165-6. [DOI] [PubMed] [Google Scholar]
- Johansson G. Effect of cholestyramine and diet on hydroxylations in the biosynthesis and metabolism of bile acids. Eur J Biochem. 1970 Dec;17(2):292–295. doi: 10.1111/j.1432-1033.1970.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Hradek G. T., Renston R. H., Wong K. Y., Karlaganis G., Paumgartner G. Autoradiographic evidence for hepatic lobular concentration gradient of bile acid derivative. Am J Physiol. 1980 Mar;238(3):G233–G237. doi: 10.1152/ajpgi.1980.238.3.G233. [DOI] [PubMed] [Google Scholar]
- Kempen H. J., De Lange J., Vos-Van Holstein M. P., Van Wachem P., Havinga R., Vonk R. J. Effect of ML-236B (compactin) on biliary excretion of bile salts and lipids, and on bile flow, in the rat. Biochim Biophys Acta. 1984 Jul 26;794(3):435–443. doi: 10.1016/0005-2760(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- Kuipers F., Dijkstra T., Havinga R., van Asselt W., Vonk R. J. Acute effects of pentobarbital-anaesthesia on bile secretion. Biochem Pharmacol. 1985 May 15;34(10):1731–1736. doi: 10.1016/0006-2952(85)90642-2. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Havinga R., Bosschieter H., Toorop G. P., Hindriks F. R., Vonk R. J. Enterohepatic circulation in the rat. Gastroenterology. 1985 Feb;88(2):403–411. doi: 10.1016/0016-5085(85)90499-8. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Nagelkerke J. F., Bakkeren H., Havinga R., Van Berkel T. J., Vonk R. J. Processing of cholesteryl ester from low-density lipoproteins in the rat. Hepatic metabolism and biliary secretion after uptake by different hepatic cell types. Biochem J. 1989 Feb 1;257(3):699–704. doi: 10.1042/bj2570699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li A. C., Tanaka R. D., Callaway K., Fogelman A. M., Edwards P. A. Localization of 3-hydroxy-3-methylglutaryl CoA reductase and 3-hydroxy-3-methylglutaryl CoA synthase in the rat liver and intestine is affected by cholestyramine and mevinolin. J Lipid Res. 1988 Jun;29(6):781–796. [PubMed] [Google Scholar]
- Nagelkerke J. F., Bakkeren H. F., Kuipers F., Vonk R. J., van Berkel T. J. Hepatic processing of the cholesteryl ester from low density lipoprotein in the rat. J Biol Chem. 1986 Jul 5;261(19):8908–8913. [PubMed] [Google Scholar]
- Nervi F., Bronfman M., Allalón W., Depiereux E., Del Pozo R. Regulation of biliary cholesterol secretion in the rat. Role of hepatic cholesterol esterification. J Clin Invest. 1984 Dec;74(6):2226–2237. doi: 10.1172/JCI111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard C. J., Shepherd J. The hepatobiliary axis and lipoprotein metabolism: effects of bile acid sequestrants and ileal bypass surgery. J Lipid Res. 1982 Nov;23(8):1081–1098. [PubMed] [Google Scholar]
- Philipp B. W., Shapiro D. J. Improved methods for the assay and activation of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1979 Jul;20(5):588–593. [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of catabolism of rat and human low density lipoproteins in rats. Biochim Biophys Acta. 1982 Jan 15;710(1):7–14. doi: 10.1016/0005-2760(82)90183-7. [DOI] [PubMed] [Google Scholar]
- Princen H. M., Huijsmans C. M., Kuipers F., Vonk R. J., Kempen H. J. Ketoconazole blocks bile acid synthesis in hepatocyte monolayer cultures and in vivo in rat by inhibiting cholesterol 7 alpha-hydroxylase. J Clin Invest. 1986 Oct;78(4):1064–1071. doi: 10.1172/JCI112662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Hofstee B. Dexamethasone regulates bile acid synthesis in monolayer cultures of rat hepatocytes by induction of cholesterol 7 alpha-hydroxylase. Biochem J. 1989 Aug 15;262(1):341–348. doi: 10.1042/bj2620341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Kwekkeboom J., Kempen H. J. Assay of cholesterol 7 alpha-hydroxylase activity in rat hepatocytes in primary monolayer culture. Anal Biochem. 1988 May 15;171(1):158–165. doi: 10.1016/0003-2697(88)90137-6. [DOI] [PubMed] [Google Scholar]
- Rahman K., Coleman R. Selective biliary lipid secretion at low bile-salt-output rates in the isolated perfused rat liver. Effects of phalloidin. Biochem J. 1986 Jul 1;237(1):301–304. doi: 10.1042/bj2370301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K., Hammond T. G., Lowe P. J., Barnwell S. G., Clark B., Coleman R. Control of biliary phospholipid secretion. Effect of continuous and discontinuous infusion of taurocholate on biliary phospholipid secretion. Biochem J. 1986 Mar 1;234(2):421–427. doi: 10.1042/bj2340421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Redinger R. N., Hawkins J. W., Grace D. M. The economy of the enterohepatic circulation of bile acids in the baboon. 1. Studies of controlled enterohepatic circulation of bile acids. J Lipid Res. 1984 May;25(5):428–436. [PubMed] [Google Scholar]
- Robins S. J., Fasulo J. M., Leduc R., Patton G. M. The transport of lipoprotein cholesterol into bile: a reassessment of kinetic studies in the experimental animal. Biochim Biophys Acta. 1989 Aug 22;1004(3):327–331. doi: 10.1016/0005-2760(89)90080-5. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y. Motor functions of the intestine. Adv Vet Sci Comp Med. 1981;25:345–369. [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Kazazis D. M., Alberts A. W., Chen J. S., Huff J. W., Ness G. C. Hydroxymethylglutaryl-coenzyme A reductase-containing hepatocytes are distributed periportally in normal and mevinolin-treated rat livers. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5556–5560. doi: 10.1073/pnas.81.17.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M. Gallstones. N Engl J Med. 1968 Sep 12;279(11):588–593. doi: 10.1056/NEJM196809122791106. [DOI] [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Rates of low density lipoprotein uptake and cholesterol synthesis are regulated independently in the liver. J Lipid Res. 1985 Apr;26(4):465–472. [PubMed] [Google Scholar]
- Tavoloni N. Bile acid structure and bile formation in the guinea pig. Biochim Biophys Acta. 1986 Nov 14;879(2):186–201. doi: 10.1016/0005-2760(86)90102-5. [DOI] [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. The contribution of newly synthesized cholesterol to biliary cholesterol in the rat. J Biol Chem. 1981 Mar 10;256(5):2438–2446. [PubMed] [Google Scholar]
- Weis E. E., Barth C. A. The extracorporeal bile duct: a new model for determination of bile flow and bile composition in the intact rat. J Lipid Res. 1978 Sep;19(7):856–862. [PubMed] [Google Scholar]
- Wheeler H. O., King K. K. Biliary excretion of lecithin and cholesterol in the dog. J Clin Invest. 1972 Jun;51(6):1337–1350. doi: 10.1172/JCI106930. [DOI] [PMC free article] [PubMed] [Google Scholar]



