Abstract
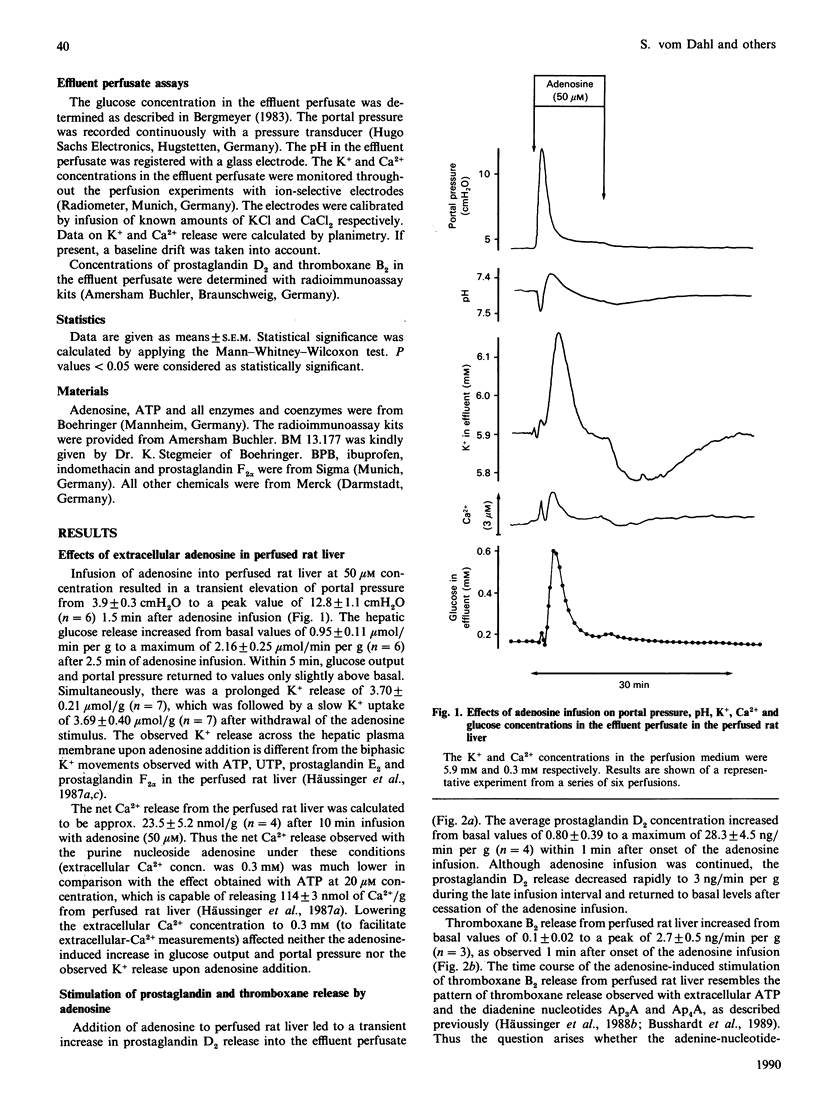
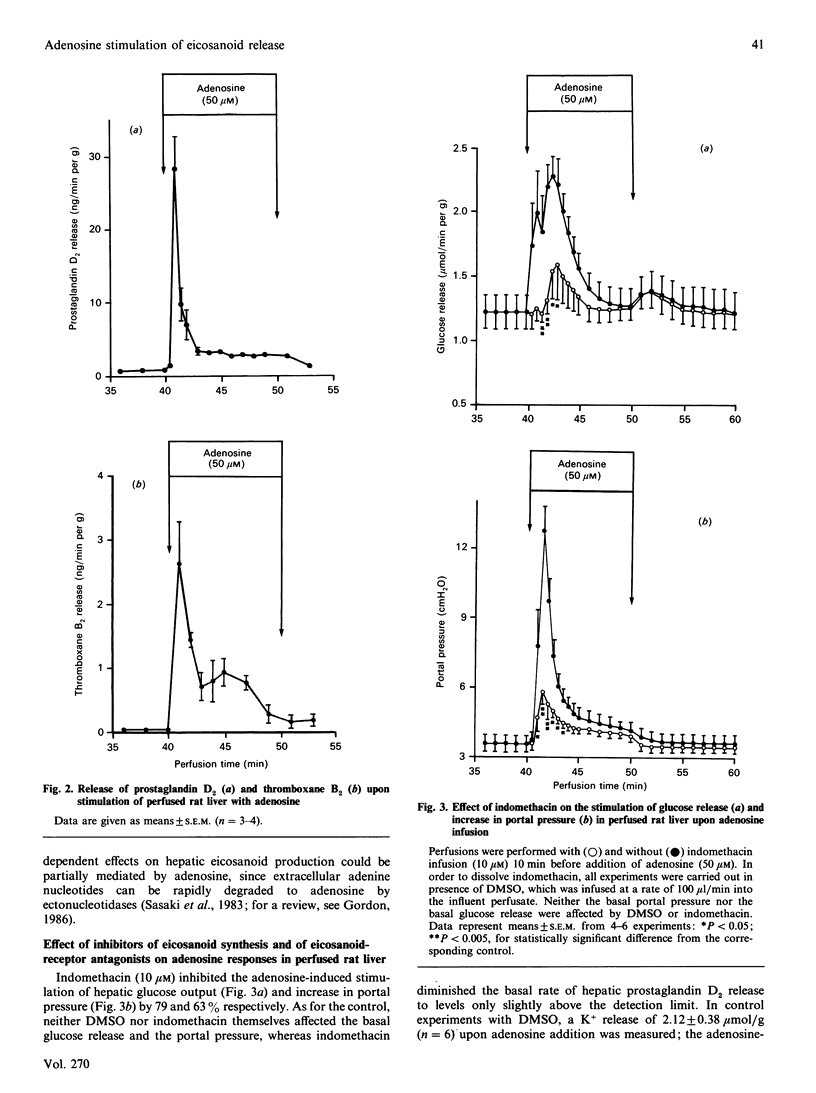
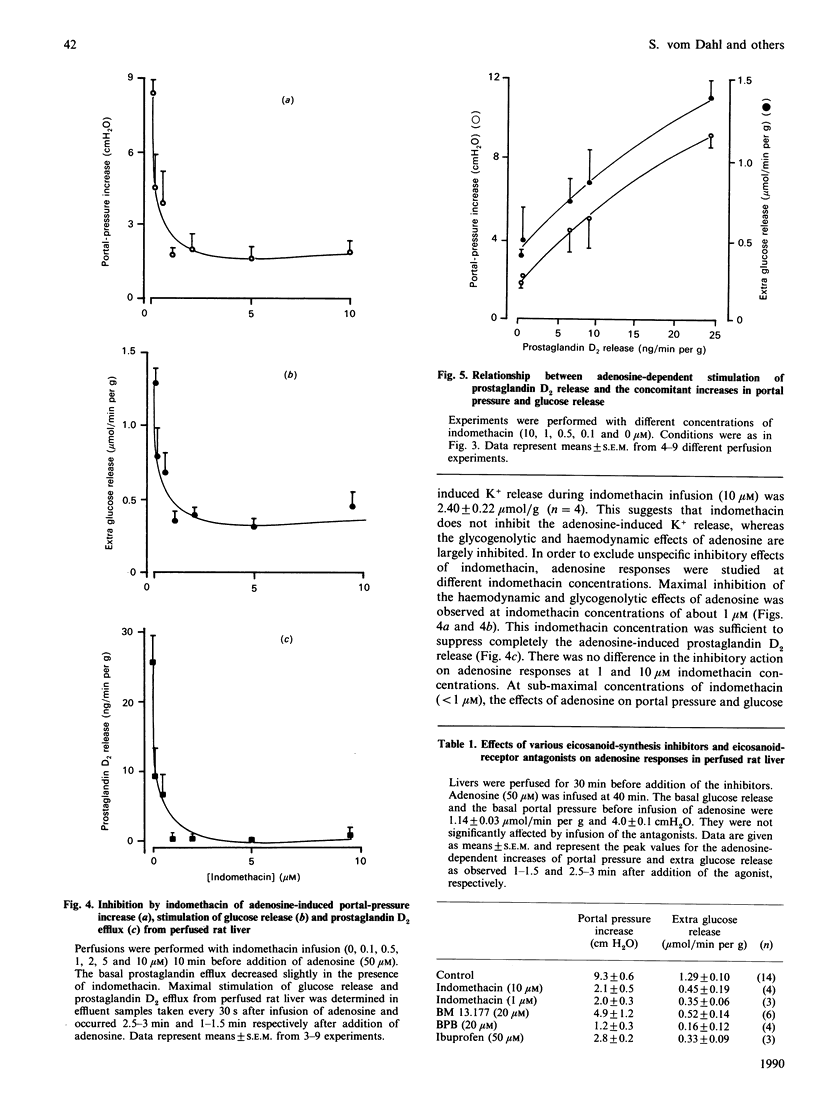
In isolated perfused rat liver, adenosine infusion (50 microM) led to increases in glucose output and portal pressure and a net K+ release of 3.7 +/- 0.21 mumol/g, which was followed by an equivalent net K+ uptake after cessation of the nucleoside infusion. These effects were accompanied by a transient stimulation of hepatic prostaglandin D2 and thromboxane B2 release. The Ca2+ release observed upon adenosine infusion (50 microM) was 23.5 +/- 5.2 nmol/g, i.e. 10-20% of the Ca2+ release observed with extracellular ATP (50 microM). Indomethacin (10 microM) prevented the adenosine-induced stimulation of glucose output and the increase in portal pressure by 79 and 63% respectively, and completely abolished the stimulation of prostaglandin D2 release. The thromboxane A2 receptor antagonist BM 13.177 (20 microM), the phospholipase A2 inhibitor 4-bromophenacyl bromide (20 microM) and the cyclo-oxygenase inhibitor ibuprofen (50 microM) also decreased the glycogenolytic and vasoconstrictive responses of the perfused rat liver upon adenosine infusion by 50-80%. When the indomethacin inhibition of adenosine-induced prostaglandin D2 release was titrated, a close correlation between prostaglandin D2 release and the metabolic and vascular responses to adenosine was observed. These findings suggest an important role for eicosanoids in mediating the nucleoside responses in the perfused rat liver. Since eicosanoids are known to be formed by non-parenchymal cells in rat liver [Decker (1985) Semin. Liver Dis. 5, 175-190], the present study gives further evidence for an important role of eicosanoids as signal molecules between the different liver cell populations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. Prostaglandin F2 alpha and the thromboxane A2 analogue ONO-11113 stimulate Ca2+ fluxes and other physiological responses in rat liver. Further evidence that prostanoids may be involved in the action of arachidonic acid and platelet-activating factor. Biochem J. 1988 Feb 1;249(3):677–685. doi: 10.1042/bj2490677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrons R., Van Schaftingen E., Hers H. G. The ability of adenosine to decrease the concentration of fructose 2,6-bisphosphate in isolated hepatocytes. A cyclic AMP-mediated effect. Biochem J. 1984 Feb 15;218(1):157–163. doi: 10.1042/bj2180157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmelin M., Decker K. Synthesis of prostanoids and cyclic nucleotides by phagocytosing rat Kupffer cells. Eur J Biochem. 1984 Jul 16;142(2):219–225. doi: 10.1111/j.1432-1033.1984.tb08274.x. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of catecholamines, ATP and ionophore A23187 on potassium and calcium movements in isolated hepatocytes. Nature. 1979 Jun 7;279(5713):544–546. doi: 10.1038/279544a0. [DOI] [PubMed] [Google Scholar]
- Busshardt E., Gerok W., Häussinger D. Regulation of hepatic parenchymal and non-parenchymal cell function by the diadenine nucleotides Ap3A and Ap4A. Biochim Biophys Acta. 1989 Feb 9;1010(2):151–159. doi: 10.1016/0167-4889(89)90155-9. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Fisher R. A., Briseno D. L., Hanahan D. J., Olson M. S. Glycogenolytic and haemodynamic responses to heat-aggregated immunoglobulin G and prostaglandin E2 in the perfused rat liver. Biochem J. 1987 Apr 15;243(2):493–498. doi: 10.1042/bj2430493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D. B., Fisher R. A., Robertson S. M., Olson M. S. Stimulation of glycogenolysis and vasoconstriction by adenosine and adenosine analogues in the perfused rat liver. Biochem J. 1987 Nov 15;248(1):35–41. doi: 10.1042/bj2480035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D. B., Robertson S. M., Olson M. S. Stimulation of glycogenolysis by adenine nucleotides in the perfused rat liver. Biochem J. 1986 Aug 1;237(3):773–780. doi: 10.1042/bj2370773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteleijn E., Kuiper J., Van Rooij H. C., Kamps J. A., Koster J. F., Van Berkel T. J. Endotoxin stimulates glycogenolysis in the liver by means of intercellular communication. J Biol Chem. 1988 May 25;263(15):6953–6955. [PubMed] [Google Scholar]
- Casteleijn E., Kuiper J., van Rooij H. C., Kamps J. A., Koster J. F., van Berkel T. J. Hormonal control of glycogenolysis in parenchymal liver cells by Kupffer and endothelial liver cells. J Biol Chem. 1988 Feb 25;263(6):2699–2703. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Christ B., Löhne R., Schmidt H., Jungermann K. Modulation of the glucagon-dependent induction of phosphoenolpyruvate carboxykinase by adenosine, but not ketone bodies or ammonia in rat hepatocyte cultures. Possible significance for the zonal heterogeneity of liver parenchyma. Biol Chem Hoppe Seyler. 1987 Dec;368(12):1579–1587. doi: 10.1515/bchm3.1987.368.2.1579. [DOI] [PubMed] [Google Scholar]
- Decker K. Eicosanoids, signal molecules of liver cells. Semin Liver Dis. 1985 May;5(2):175–190. doi: 10.1055/s-2008-1063921. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Robertson S. M., Olson M. S. Stimulation of glycogenolysis and vasoconstriction in the perfused rat liver by the thromboxane A2 analogue U-46619. J Biol Chem. 1987 Apr 5;262(10):4631–4638. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer L. J., Lowenstein J. M. Effects of adenosine and adenosine analogues on glycogen metabolism in isolated rat hepatocytes. Biochem Pharmacol. 1986 Dec 15;35(24):4529–4536. doi: 10.1016/0006-2952(86)90775-6. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Busshardt E., Stehle T., Stoll B., Wettstein M., Gerok W. Stimulation of thromboxane release by extracellular UTP and ATP from perfused rat liver. Role of icosanoids in mediating the nucleotide responses. Eur J Biochem. 1988 Dec 1;178(1):249–256. doi: 10.1111/j.1432-1033.1988.tb14450.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Gerok W. Actions of extracellular UTP and ATP in perfused rat liver. A comparative study. Eur J Biochem. 1987 Aug 17;167(1):65–71. doi: 10.1111/j.1432-1033.1987.tb13304.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Gerok W. Effects of leukotrienes and the thromboxane A2 analogue U-46619 in isolated perfused rat liver. Metabolic, hemodynamic and ion-flux responses. Biol Chem Hoppe Seyler. 1988 Feb;369(2):97–107. doi: 10.1515/bchm3.1988.369.1.97. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Gerok W., Sies H. Perivascular nerve stimulation and phenylephrine responses in rat liver. Metabolic effects, Ca2+ and K+ fluxes. Eur J Biochem. 1987 Feb 16;163(1):197–203. doi: 10.1111/j.1432-1033.1987.tb10755.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stehle T. Hepatocyte heterogeneity in response to icosanoids. The perivenous scavenger cell hypothesis. Eur J Biochem. 1988 Aug 1;175(2):395–403. doi: 10.1111/j.1432-1033.1988.tb14209.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Tran-Thi T. A., Decker K., Gerok W. Prostaglandin responses in isolated perfused rat liver: Ca2+ and K+ fluxes, hemodynamic and metabolic effects. Biol Chem Hoppe Seyler. 1987 Nov;368(11):1509–1513. doi: 10.1515/bchm3.1987.368.2.1509. [DOI] [PubMed] [Google Scholar]
- Ismail N. A., Hems D. A. Effects of adenosine on glucose and lipid metabolism and hepatic blood flow. Biochem Pharmacol. 1978 May 1;27(9):1341–1345. doi: 10.1016/0006-2952(78)90117-x. [DOI] [PubMed] [Google Scholar]
- Iwai M., Gardemann A., Püschel G., Jungermann K. Potential role for prostaglandin F2 alpha, D2, E2 and thromboxane A2 in mediating the metabolic and hemodynamic actions of sympathetic nerves in perfused rat liver. Eur J Biochem. 1988 Jul 15;175(1):45–50. doi: 10.1111/j.1432-1033.1988.tb14164.x. [DOI] [PubMed] [Google Scholar]
- Iwai M., Jungermann K. Possible involvement of eicosanoids in the actions of sympathetic hepatic nerves on carbohydrate metabolism and hemodynamics in perfused rat liver. FEBS Lett. 1987 Aug 31;221(1):155–160. doi: 10.1016/0014-5793(87)80371-x. [DOI] [PubMed] [Google Scholar]
- Lapointe D. S., Olson M. S. Platelet-activating factor-stimulated hepatic glycogenolysis is not mediated through cyclooxygenase-derived metabolites of arachidonic acid. J Biol Chem. 1989 Jul 25;264(21):12130–12133. [PubMed] [Google Scholar]
- Lavoinne A., Buc H. A., Claeyssens S., Pinosa M., Matray F. The mechanism by which adenosine decreases gluconeogenesis from lactate in isolated rat hepatocytes. Biochem J. 1987 Sep 1;246(2):449–454. doi: 10.1042/bj2460449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Abe A., Sakagami T. Ecto-5'-nucleotidase does not catalyze vectorial production of adenosine in the perfused rat liver. J Biol Chem. 1983 Jun 10;258(11):6947–6951. [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Staddon J. M., McGivan J. D. Effects of ATP and adenosine addition on activity of oxoglutarate dehydrogenase and the concentration of cytoplasmic free Ca2+ in rat hepatocytes. Eur J Biochem. 1985 Sep 16;151(3):567–572. doi: 10.1111/j.1432-1033.1985.tb09141.x. [DOI] [PubMed] [Google Scholar]
- Stanley J. C., Markovic J., Gutknecht A. M., Lozeman F. J. Stimulation of glycogenolysis in isolated hepatocytes by adenosine and one of its analogues is inhibited by caffeine. Biochem J. 1987 Nov 1;247(3):779–783. doi: 10.1042/bj2470779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier K., Pill J., Müller-Beckmann B., Schmidt F. H., Witte E. C., Wolff H. P., Patscheke H. The pharmacological profile of the thromboxane A2 antagonist BM 13.177. A new anti-platelet and anti-thrombotic drug. Thromb Res. 1984 Aug 15;35(4):379–395. doi: 10.1016/0049-3848(84)90230-5. [DOI] [PubMed] [Google Scholar]
- Theen J., Gilboe D. P., Nuttall F. Q. Liver glycogen synthase and phosphorylase changes in vivo with hypoxia and anesthetics. Am J Physiol. 1982 Sep;243(3):E182–E187. doi: 10.1152/ajpendo.1982.243.3.E182. [DOI] [PubMed] [Google Scholar]
- Tran-Thi T. A., Gyufko K., Häussinger D., Decker K. Net prostaglandin release by perfused rat liver after stimulation with phorbol 12-myristate 13-acetate. J Hepatol. 1988 Apr;6(2):151–157. doi: 10.1016/s0168-8278(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Tran-Thi T. A., Häussinger D., Gyufko K., Decker K. Stimulation of prostaglandin release by Ca2+-mobilizing agents from the perfused rat liver. A comparative study on the action of ATP, UTP, phenylephrine, vasopressin and nerve stimulation. Biol Chem Hoppe Seyler. 1988 Jan;369(1):65–68. doi: 10.1515/bchm3.1988.369.1.65. [DOI] [PubMed] [Google Scholar]
- Volwerk J. J., Pieterson W. A., de Haas G. H. Histidine at the active site of phospholipase A2. Biochemistry. 1974 Mar 26;13(7):1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- Wölfle D., Schmidt H., Jungermann K. Short-term modulation of glycogen metabolism, glycolysis and gluconeogenesis by physiological oxygen concentrations in hepatocyte cultures. Eur J Biochem. 1983 Oct 3;135(3):405–412. doi: 10.1111/j.1432-1033.1983.tb07667.x. [DOI] [PubMed] [Google Scholar]


