Abstract
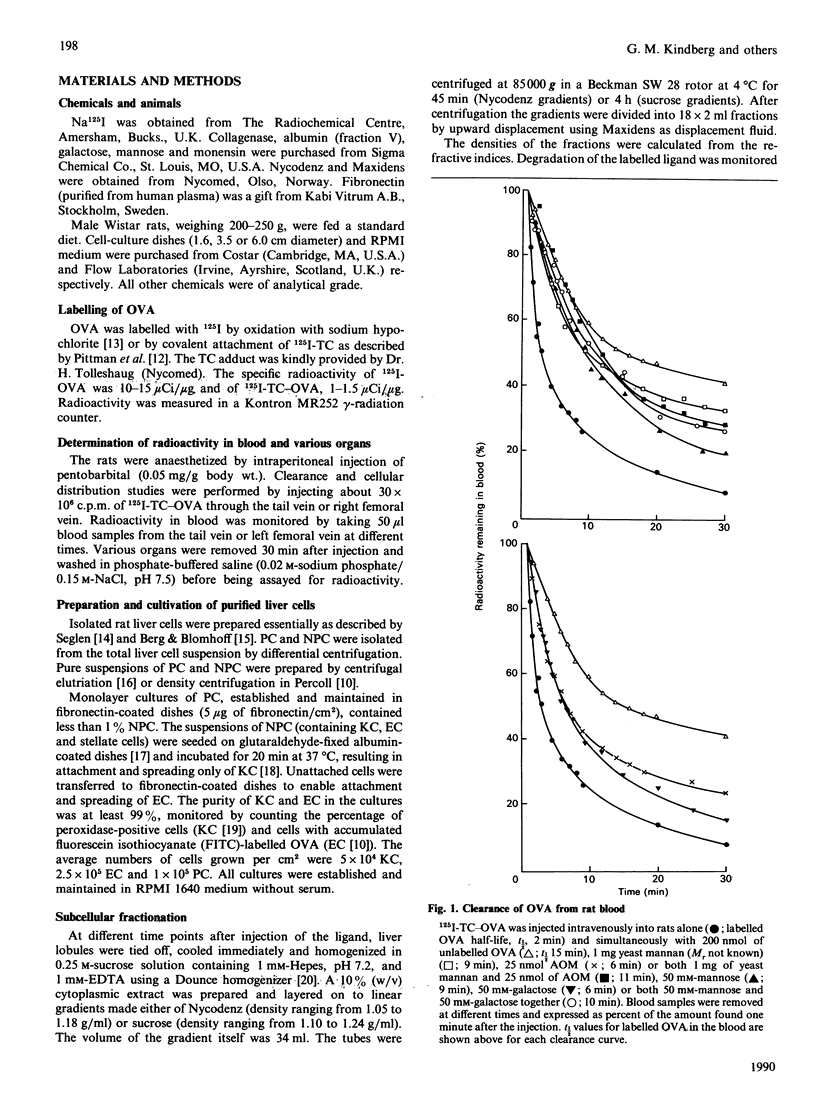
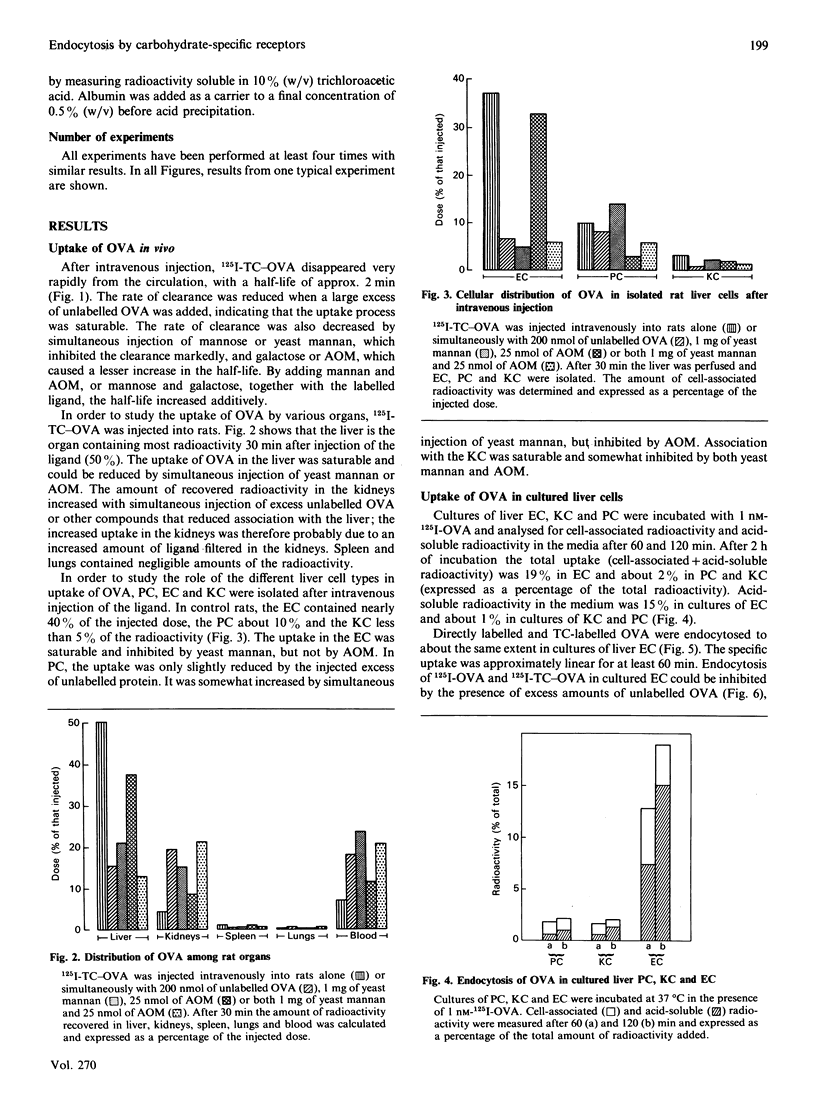
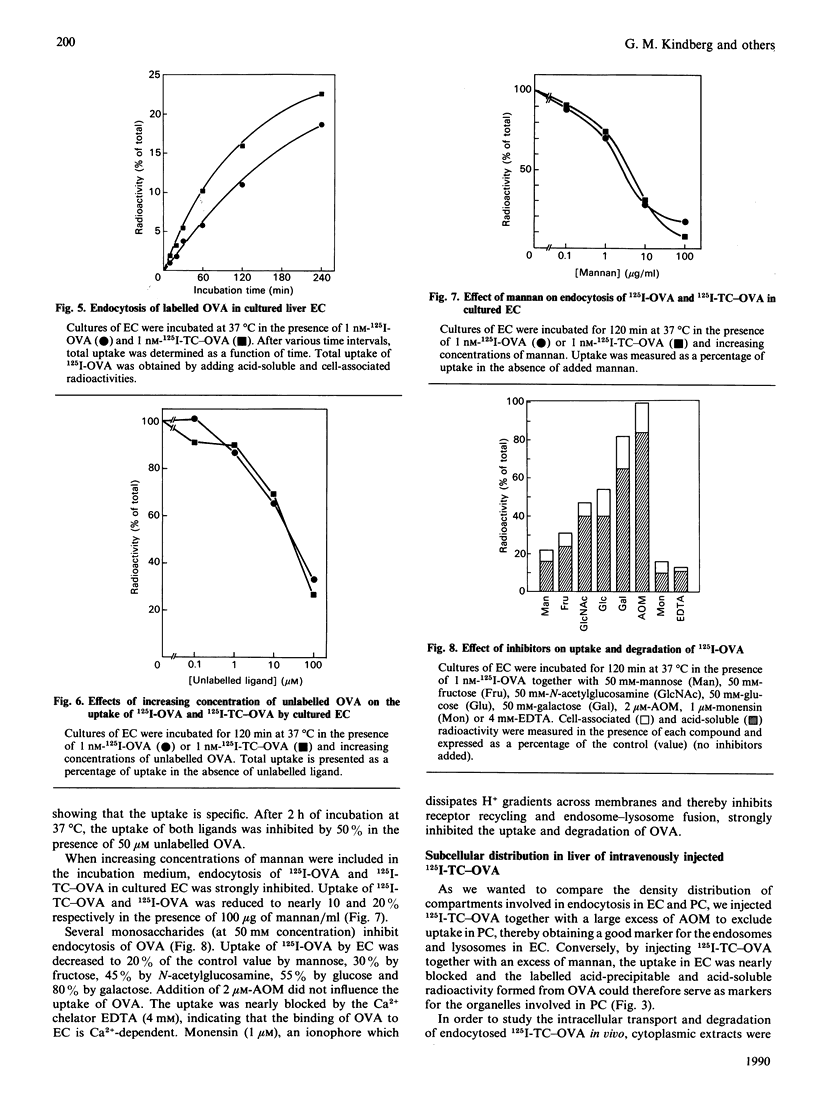
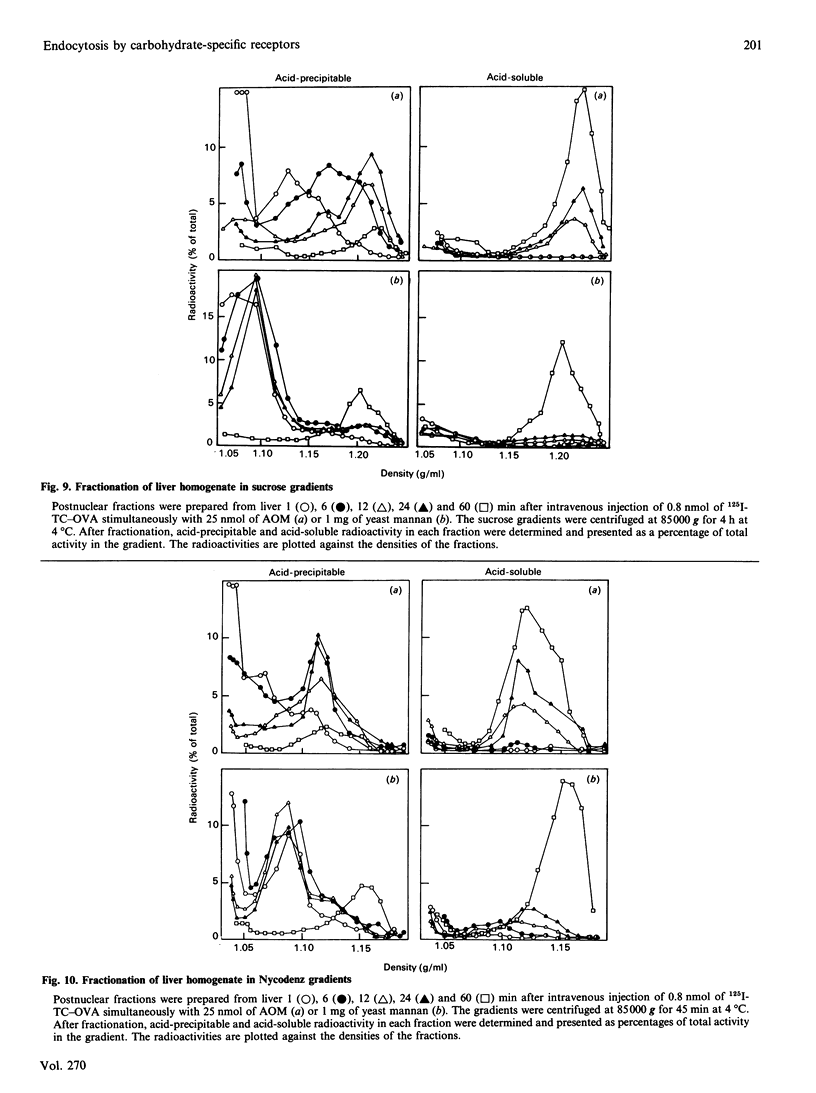
1. The uptake of ovalbumin (OVA) in rat liver parenchymal cells (PC) and non-parenchymal cells was studied in vivo and in vitro in order to compare the cellular expression of glycoprotein receptors and the kinetics of intracellular transport of ligand endocytosed by these receptors. 2. Ovalbumin was labelled with 125I or with 125I-tyramine-cellobiose (125I-TC). By using 125I-TC-OVA the labelled degradation products were trapped in the cells. 3. 125I-TC-OVA was rapidly cleared from blood mainly by receptor-mediated uptake in the liver. At 30 min after injection, 50% of the ligand was recovered in the liver. The endothelial cells (EC) and the PC were the predominant cell types responsible for uptake. 4. The uptake in PC was strongly inhibited by asialo-orosomucoid (AOM), but not by mannan, indicating that the uptake in these cells was mediated by the galactose receptor and not by the mannose receptor. This finding is compatible with the observation that a proportion of the OVA contains terminal galactose residues in the carbohydrate moiety. 5. In vitro uptake of OVA in cultured EC was saturable and inhibited by mannan, mannose, fructose, N-acetylglucosamine, EDTA or monensin, but not by galactose or AOM. The uptake of OVA in these cells was therefore mediated by the mannose receptor. 6. To label the organelles involved in endocytosis in PC and EC, 125I-TC-OVA was injected intravenously together with an excess of either AOM or mannan. In this way the labelled ligand could be directed selectively to EC or PC respectively. Subcellular fractionation of total liver in sucrose and Nycodenz gradients revealed that in EC the intracellular transport of OVA is so fast that endocytosed ligand accumulates and thus increases the density of the lysosomes. Conversely, in PC transfer of ligand is slower, with the result that accumulation of undegraded ligand in the lysosomes does not occur. These findings are interpreted to mean that in EC the rate-limiting step of handling of endocytosed ligand is intralysosomal degradation, whereas in PC the rate-limiting step is transport of ligand to the lysosomes. 7. Altogether, these findings suggest that endocytosis of OVA by the liver EC and PC is mediated by mannose and galactose receptors respectively, and that the kinetics of intracellular transport of OVA differ in the two cell types.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Bell C. E., Sly W. S. Human beta-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978 Sep;15(1):269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Berg T., Kindberg G. M., Ford T., Blomhoff R. Intracellular transport of asialoglycoproteins in rat hepatocytes. Evidence for two subpopulations of lysosomes. Exp Cell Res. 1985 Dec;161(2):285–296. doi: 10.1016/0014-4827(85)90086-2. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Blomhoff H. K., Tolleshaug H., Christensen T. B., Berg T. Uptake and degradation of bovine testes beta-galactosidase by parenchymal and nonparenchymal rat liver cells. Int J Biochem. 1985;17(12):1321–1328. doi: 10.1016/0020-711x(85)90055-2. [DOI] [PubMed] [Google Scholar]
- Eskild W., Kindberg G. M., Smedsrod B., Blomhoff R., Norum K. R., Berg T. Intracellular transport of formaldehyde-treated serum albumin in liver endothelial cells after uptake via scavenger receptors. Biochem J. 1989 Mar 1;258(2):511–520. doi: 10.1042/bj2580511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiwanger R. S., Hill R. L. The ligand binding specificity and tissue localization of a rat alveolar macrophage lectin. J Biol Chem. 1986 Nov 25;261(33):15696–15702. [PubMed] [Google Scholar]
- Hildenbrandt G. R., Aronson N. N., Jr Endocytosis of bovine lactoperoxidase by two carbohydrate-specific receptors in rat liver. Arch Biochem Biophys. 1985 Feb 15;237(1):1–10. doi: 10.1016/0003-9861(85)90247-4. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Wilson G., Ashwell G., Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. I. Distribution of 125I-ligands among the liver cell types. J Cell Biol. 1979 Oct;83(1):47–64. doi: 10.1083/jcb.83.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson S., Berg T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochem J. 1989 Feb 1;257(3):651–656. doi: 10.1042/bj2570651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenseter M. S., Blomhoff R., Drevon C. A., Kindberg G. M., Norum K. R., Berg T. Uptake of LDL in parenchymal and non-parenchymal rabbit liver cells in vivo. LDL uptake is increased in endothelial cells in cholesterol-fed rabbits. Biochem J. 1988 Sep 1;254(2):443–448. doi: 10.1042/bj2540443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redshaw M. R., Lynch S. S. An improved method for the preparation of iodinated antigens for radioimmunoassay. J Endocrinol. 1974 Mar;60(3):527–528. doi: 10.1677/joe.0.0600527. [DOI] [PubMed] [Google Scholar]
- Schlesinger P. H., Doebber T. W., Mandell B. F., White R., DeSchryver C., Rodman J. S., Miller M. J., Stahl P. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochem J. 1978 Oct 15;176(1):103–109. doi: 10.1042/bj1760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Campbell E. J., Senior R. M., Stahl P. D. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982 Dec;32(6):423–431. [PubMed] [Google Scholar]
- Smedsrød B., Einarsson M. Clearance of tissue plasminogen activator by mannose and galactose receptors in the liver. Thromb Haemost. 1990 Feb 19;63(1):60–66. [PubMed] [Google Scholar]
- Smedsrød B., Einarsson M., Pertoft H. Tissue plasminogen activator is endocytosed by mannose and galactose receptors of rat liver cells. Thromb Haemost. 1988 Jun 16;59(3):480–484. [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol. 1985 Aug;38(2):213–230. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982 Apr;93(1):49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Blomhoff R. Uptake of mannose-terminated glycoproteins in isolated rat liver cells. Evidence for receptor-mediated endocytosis in hepatocytes. Biochem J. 1984 Oct 1;223(1):151–160. doi: 10.1042/bj2230151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T. The effect of leupeptin on intracellular digestion of asialofetuin in rat hepatocytes. Exp Cell Res. 1981 Jul;134(1):207–217. doi: 10.1016/0014-4827(81)90478-x. [DOI] [PubMed] [Google Scholar]


