Abstract
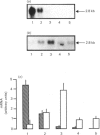
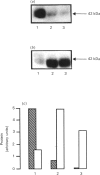
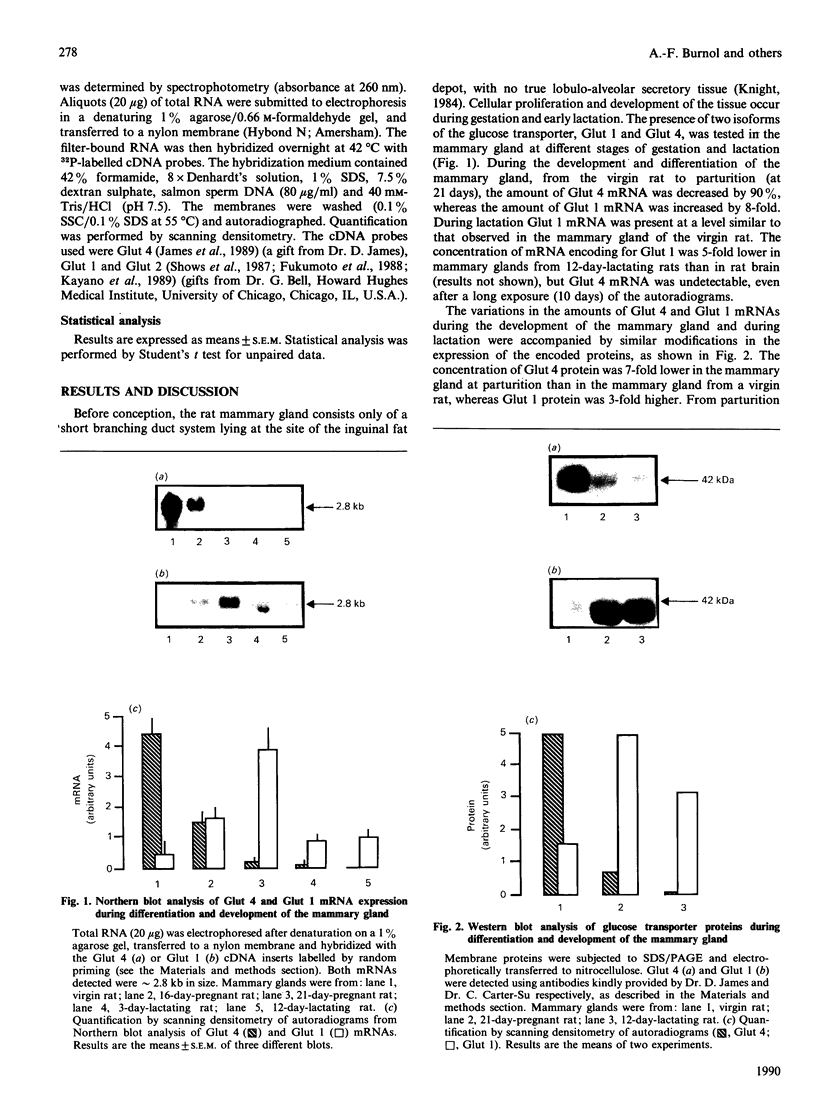
The expression of different glucose transporter isoforms was measured during the development and differentiation of the rat mammary gland. Before conception, when the mammary gland is mainly composed of adipocytes, Glut 4 and Glut 1 mRNAs and proteins were present. During pregnancy, the expression of Glut 4 decreased progressively, whereas that of Glut 1 increased. In the lactating mammary gland only Glut 1 was present, and was expressed at a high level. The absence of Glut 4 suggests that glucose transport is not regulated by insulin in the lactating rat mammary gland.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter M. A., Coore H. G. The mode of regulation of pyruvate dehydrogenase of lactating rat mammary gland. Effects of starvation and insulin. Biochem J. 1978 Aug 15;174(2):553–561. doi: 10.1042/bj1740553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Burnol A. F., Ebner S., Ferré P., Girard J. Regulation by insulin of glucose metabolism in mammary gland of anaesthetized lactating rats. Stimulation of phosphofructokinase-1 by fructose 2,6-bisphosphate and activation of acetyl-CoA carboxylase. Biochem J. 1988 Aug 15;254(1):11–14. doi: 10.1042/bj2540011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Djiane J., Durand P., Kelly P. A. Evolution of prolactin receptors in rabbit mammary gland during pregnancy and lactation. Endocrinology. 1977 May;100(5):1348–1356. doi: 10.1210/endo-100-5-1348. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Herreros A., Birnbaum M. J. The regulation by insulin of glucose transporter gene expression in 3T3 adipocytes. J Biol Chem. 1989 Jun 15;264(17):9885–9890. [PubMed] [Google Scholar]
- Hiraki Y., Rosen O. M., Birnbaum M. J. Growth factors rapidly induce expression of the glucose transporter gene. J Biol Chem. 1988 Sep 25;263(27):13655–13662. [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Kilgour E., Vernon R. G. Tissue-specific changes in the ability of insulin and noradrenaline to activate pyruvate dehydrogenase in vivo during lactation in the rat. Biochem J. 1987 Apr 1;243(1):69–74. doi: 10.1042/bj2430069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Cordera R., Olefsky J. M. Substrate specificities of insulin and epidermal growth factor receptor kinases. Biochem Biophys Res Commun. 1985 Feb 28;127(1):254–263. doi: 10.1016/s0006-291x(85)80152-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNeillie E. M., Zammit V. A. Regulation of acetyl-CoA carboxylase in rat mammary gland. Effects of starvation and of insulin and prolactin deficiency on the fraction of the enzyme in the active form in vivo. Biochem J. 1982 Apr 15;204(1):273–280. doi: 10.1042/bj2040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes. 1990 Jan;39(1):6–11. doi: 10.2337/diacare.39.1.6. [DOI] [PubMed] [Google Scholar]
- Munday M. R., Williamson D. H. Insulin activation of lipogenesis in isolated mammary acini from lactating rats fed on a high-fat diet. Evidence that acetyl-CoA carboxylase is a site of action. Biochem J. 1987 Mar 15;242(3):905–911. doi: 10.1042/bj2420905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T., Kuhn N. J. Arteriovenous glucose differences across the mammary gland of the fed, starved, and re-fed lactating rat. Biochem J. 1986 Oct 15;239(2):269–274. doi: 10.1042/bj2390269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser C. G., Topper Y. J. Changes in the rate of carrier-mediated glucose transport by mouse mammary epithelial cells during ontogeny: hormone dependence delineated in vitro. Endocrinology. 1986 Jul;119(1):91–96. doi: 10.1210/endo-119-1-91. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Usher P., Flier J. S. Platelet-derived growth factor regulates glucose transporter expression. J Biol Chem. 1988 Nov 15;263(32):16523–16526. [PubMed] [Google Scholar]
- Shows T. B., Eddy R. L., Byers M. G., Fukushima Y., Dehaven C. R., Murray J. C., Bell G. I. Polymorphic human glucose transporter gene (GLUT) is on chromosome 1p31.3----p35. Diabetes. 1987 Apr;36(4):546–549. doi: 10.2337/diab.36.4.546. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Threadgold L. C., Coore H. G., Kuhn N. J. Monosaccharide transport into lactating-rat mammary acini. Biochem J. 1982 May 15;204(2):493–501. doi: 10.1042/bj2040493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threadgold L. C., Kuhn N. J. Monosaccharide transport in the mammary gland of the intact lactating rat. Biochem J. 1984 Feb 15;218(1):213–219. doi: 10.1042/bj2180213. [DOI] [PMC free article] [PubMed] [Google Scholar]