Abstract
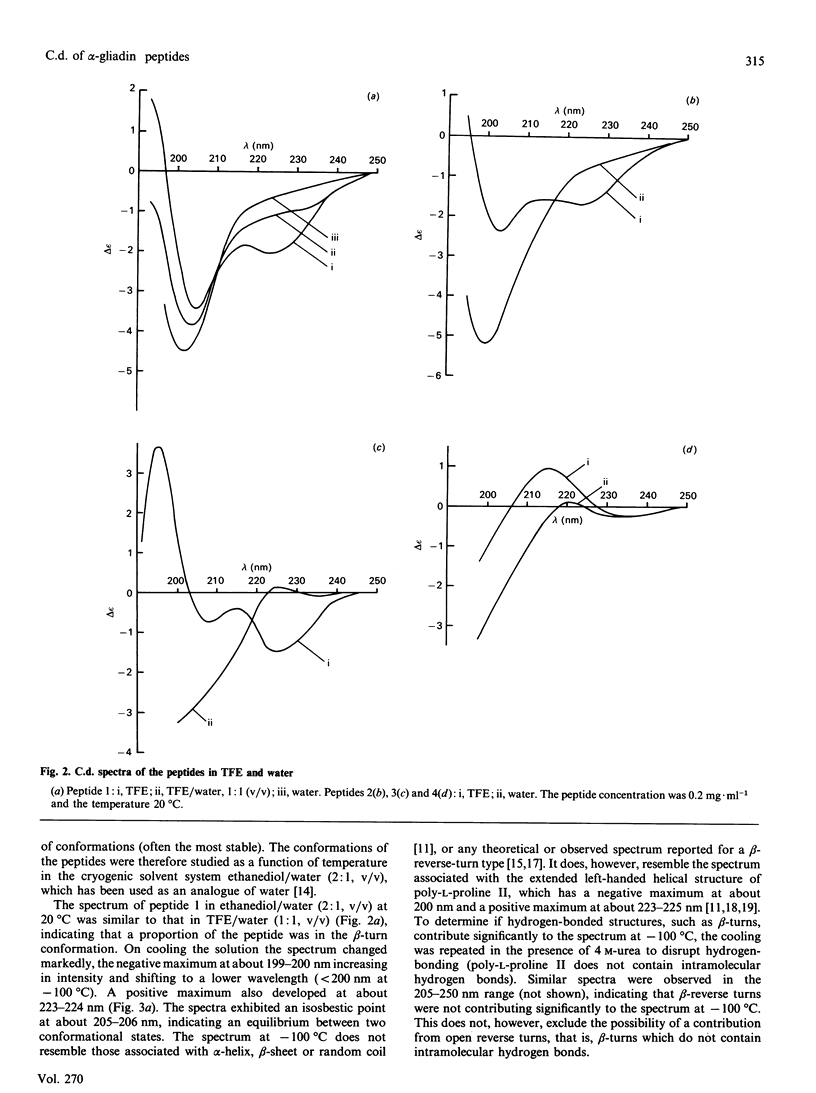
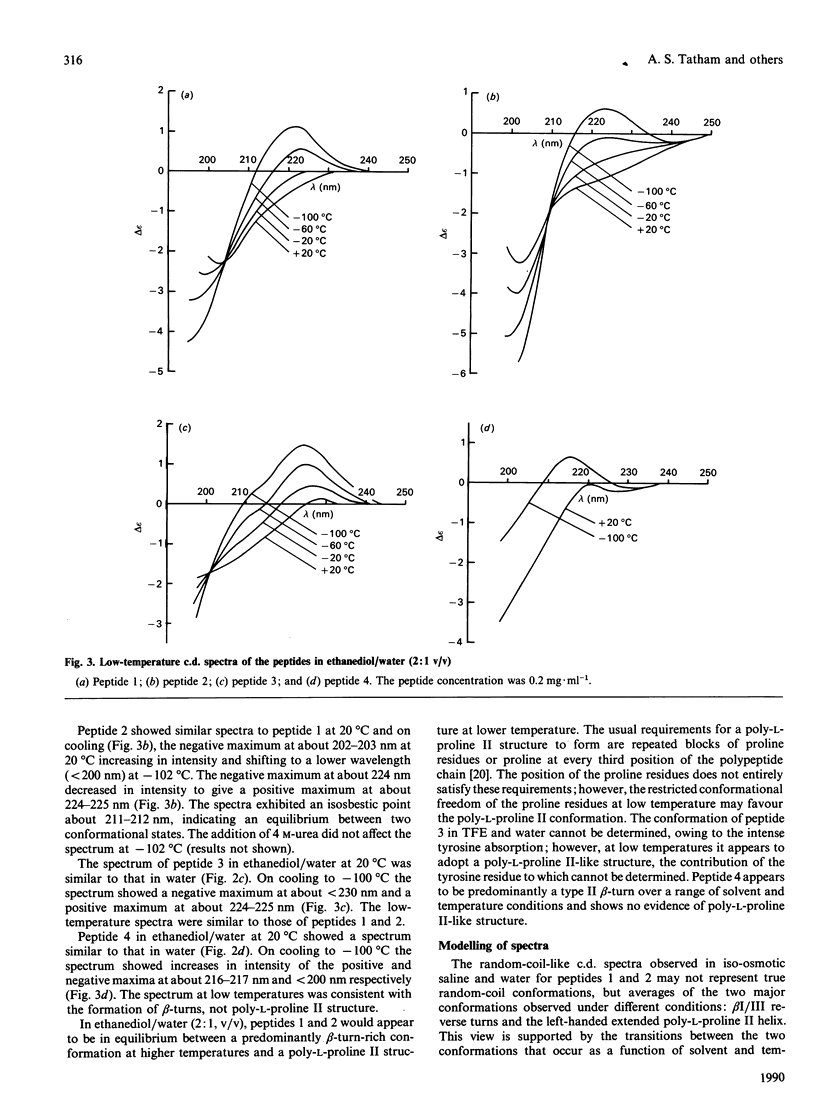
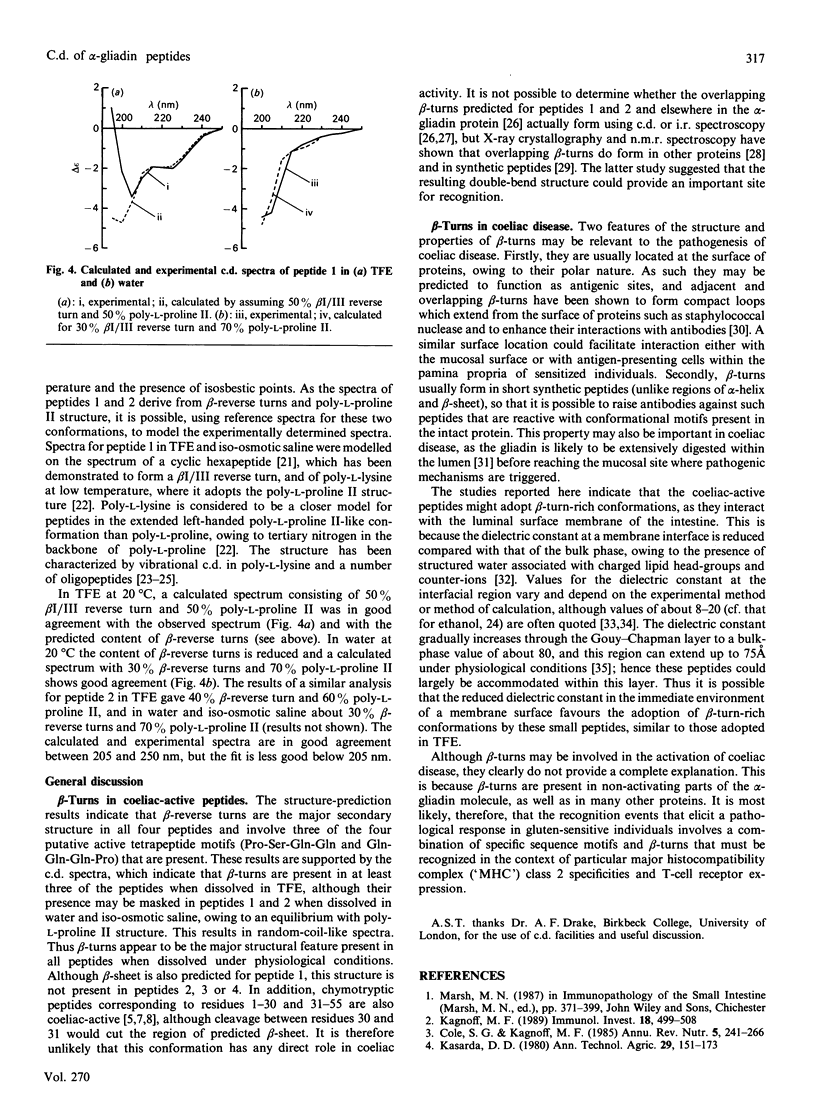
The structures of four peptides corresponding to parts of the coeliac-activating protein A-gliadin were studied by structure prediction and c.d. spectroscopy. Three of the peptides corresponded to parts of the coeliac-activating N-terminal region (residues 3-55, 3-19 and 39-45) and contained two tetrapeptide motifs common to all coeliac-active regions (Pro-Ser-Gln-Gln and Gln-Gln-Gln-Pro). The Pro-Ser-Gln-Gln sequence was also present in the fourth peptide, on the basis of the C-terminal part of the molecule (211-217). These studies showed that beta-reverse turns were the predominant structural feature in all peptides and were predominantly of type I/III in two of the N-terminal peptides and type II in the C-terminal peptide. These turns form when the peptide is dissolved in solvents of low dielectric constant (trifluoroethanol) and high dielectric constant (water and iso-osmotic saline), although their presence in the N-terminal peptides may be masked in the latter solvents due to equilibrium with a poly-L-proline II structure favoured at lower temperatures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown F. R., 3rd, Di Corato A., Lorenzi G. P., Blout E. R. Synthesis and structural studies of two collagen analogues: poly (L-prolyl-L-seryl-glycyl) and poly (L-prolyl-L-alanyl-glycyl). J Mol Biol. 1972 Jan 14;63(1):85–99. doi: 10.1016/0022-2836(72)90523-2. [DOI] [PubMed] [Google Scholar]
- Bruce G., Woodley J. F., Swan C. H. Breakdown of gliadin peptides by intestinal brush borders from coeliac patients. Gut. 1984 Sep;25(9):919–924. doi: 10.1136/gut.25.9.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush C. A., Sarkar S. K., Kopple K. D. Circular dichroism of beta turns in peptides and proteins. Biochemistry. 1978 Nov 14;17(23):4951–4954. doi: 10.1021/bi00616a015. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cole S. G., Kagnoff M. F. Celiac disease. Annu Rev Nutr. 1985;5:241–266. doi: 10.1146/annurev.nu.05.070185.001325. [DOI] [PubMed] [Google Scholar]
- D'Arrigo J. S. Screening of membrane surface charges by divalent cations: an atomic representation. Am J Physiol. 1978 Sep;235(3):C109–C117. doi: 10.1152/ajpcell.1978.235.3.C109. [DOI] [PubMed] [Google Scholar]
- Drake A. F., Siligardi G., Gibbons W. A. Reassessment of the electronic circular dichroism criteria for random coil conformations of poly(L-lysine) and the implications for protein folding and denaturation studies. Biophys Chem. 1988 Aug;31(1-2):143–146. doi: 10.1016/0301-4622(88)80019-x. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Lerner R. A., Wright P. E. The physical basis for induction of protein-reactive antipeptide antibodies. Annu Rev Biophys Biophys Chem. 1988;17:305–324. doi: 10.1146/annurev.bb.17.060188.001513. [DOI] [PubMed] [Google Scholar]
- Gierasch L. M., Deber C. M., Madison V., Niu C. H., Blout E. R. Conformations of (X-L-Pro-Y)2 cyclic hexapeptides. Preferred beta-turn conformers and implications for beta turns in proteins. Biochemistry. 1981 Aug 4;20(16):4730–4738. doi: 10.1021/bi00519a032. [DOI] [PubMed] [Google Scholar]
- Isogai Y., Némethy G., Rackovsky S., Leach S. J., Scheraga H. A. Characterization of multiple bends in proteins. Biopolymers. 1980 Jun;19(6):1183–1210. doi: 10.1002/bip.1980.360190607. [DOI] [PubMed] [Google Scholar]
- Jenness D. D., Sprecher C., Johnson W. C., Jr Circular dichroism of collagen, gelatin, and poly(proline) II in the vacuum ultraviolet. Biopolymers. 1976 Mar;15(3):513–521. doi: 10.1002/bip.1976.360150308. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F., Austin R. K., Hubert J. J., Bernardin J. E., Kasarda D. D. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med. 1984 Nov 1;160(5):1544–1557. doi: 10.1084/jem.160.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Austin R. K., Johnson H. C., Bernardin J. E., Dietler M. D., Kasarda D. D. Celiac sprue: correlation with murine T cell responses to wheat gliadin components. J Immunol. 1982 Dec;129(6):2693–2697. [PubMed] [Google Scholar]
- Kagnoff M. F. Immunopathogenesis of celiac disease. Immunol Invest. 1989 Jan-May;18(1-4):499–508. doi: 10.3109/08820138909112259. [DOI] [PubMed] [Google Scholar]
- Kasarda D. D., Okita T. W., Bernardin J. E., Baecker P. A., Nimmo C. C., Lew E. J., Dietler M. D., Greene F. C. Nucleic acid (cDNA) and amino acid sequences of alpha-type gliadins from wheat (Triticum aestivum). Proc Natl Acad Sci U S A. 1984 Aug;81(15):4712–4716. doi: 10.1073/pnas.81.15.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini M. G., Freedman T. B., Nafie L. A. Vibrational circular dichroism spectra of three conformationally distinct states and an unordered state of poly(L-lysine) in deuterated aqueous solution. Biopolymers. 1986 Sep;25(9):1751–1765. doi: 10.1002/bip.360250915. [DOI] [PubMed] [Google Scholar]
- Reed J., Hull W. E., von der Lieth C. W., Kübler D., Suhai S., Kinzel V. Secondary structure of the Arg-Gly-Asp recognition site in proteins involved in cell-surface adhesion. Evidence for the occurrence of nested beta-bends in the model hexapeptide GRGDSP. Eur J Biochem. 1988 Dec 1;178(1):141–154. doi: 10.1111/j.1432-1033.1988.tb14439.x. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Gierasch L. M., Smith J. A. Turns in peptides and proteins. Adv Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- Shibata S., Asakura J., Isemura T., Isemura S., Saitoh E., Sanada K. Conformational study of the basic proline-rich polypeptides from human parotid saliva. Int J Pept Protein Res. 1984 Feb;23(2):158–165. doi: 10.1111/j.1399-3011.1984.tb02706.x. [DOI] [PubMed] [Google Scholar]
- Tatham A. S., Drake A. F., Shewry P. R. Conformational studies of a synthetic peptide corresponding to the repeat motif of C hordein. Biochem J. 1989 Apr 15;259(2):471–476. doi: 10.1042/bj2590471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidhyanathan V. S. The computation of concentration and dielectric profiles in interfacial inhomogeneous regions, and the relation between surface charge density and surface potentials. Prog Clin Biol Res. 1985;172A:549–559. [PubMed] [Google Scholar]
- Wieser H., Belitz H. D., Ashkenazi A. Amino-acid sequence of the coeliac active gliadin peptide B 3142. Z Lebensm Unters Forsch. 1984 Nov;179(5):371–376. doi: 10.1007/BF01043432. [DOI] [PubMed] [Google Scholar]
- Wieser H., Belitz H. D., Idar D., Ashkenazi A. Coeliac activity of the gliadin peptides CT-1 and CT-2. Z Lebensm Unters Forsch. 1986 Feb;182(2):115–117. doi: 10.1007/BF01454241. [DOI] [PubMed] [Google Scholar]
- de Ritis G., Auricchio S., Jones H. W., Lew E. J., Bernardin J. E., Kasarda D. D. In vitro (organ culture) studies of the toxicity of specific A-gliadin peptides in celiac disease. Gastroenterology. 1988 Jan;94(1):41–49. doi: 10.1016/0016-5085(88)90607-5. [DOI] [PubMed] [Google Scholar]


