Abstract
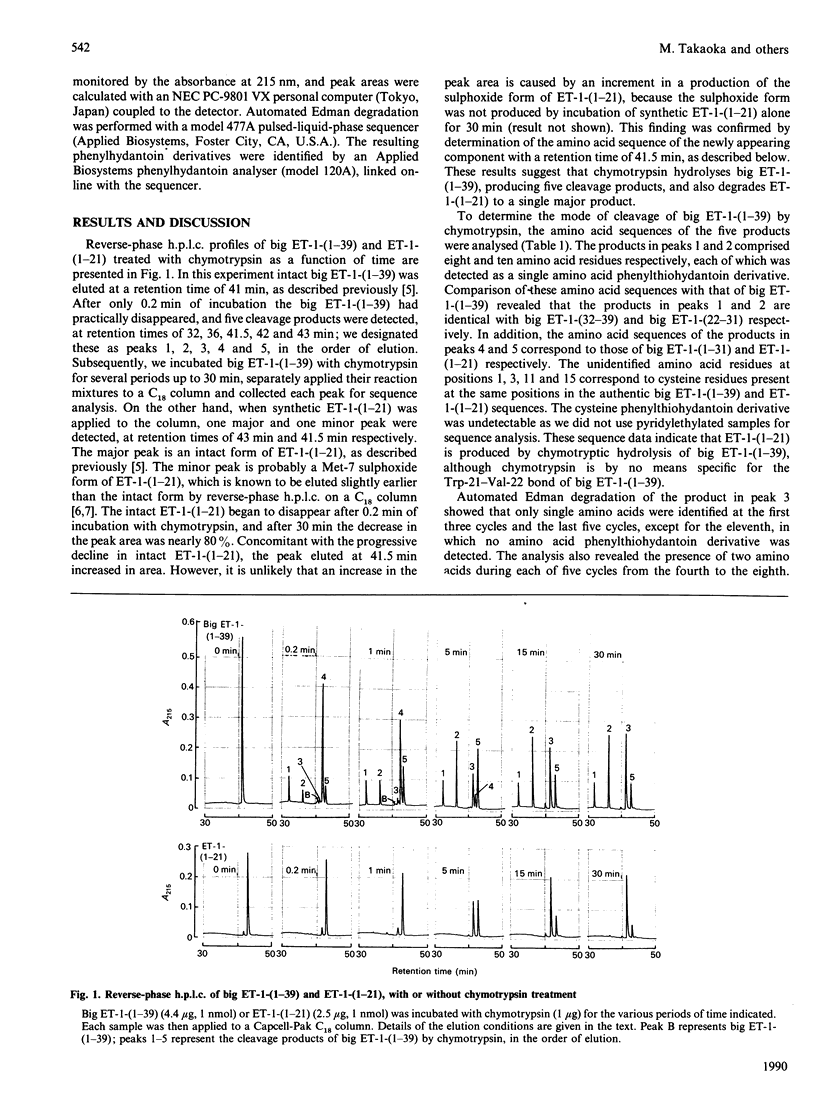
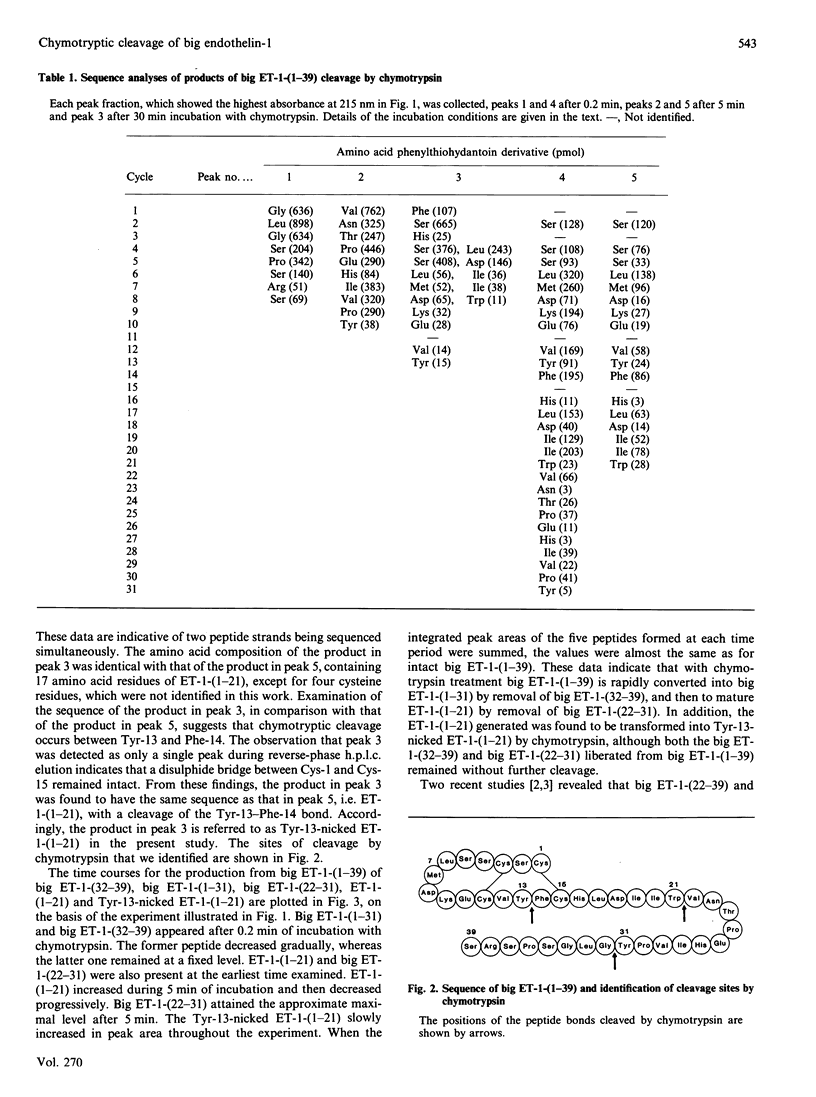
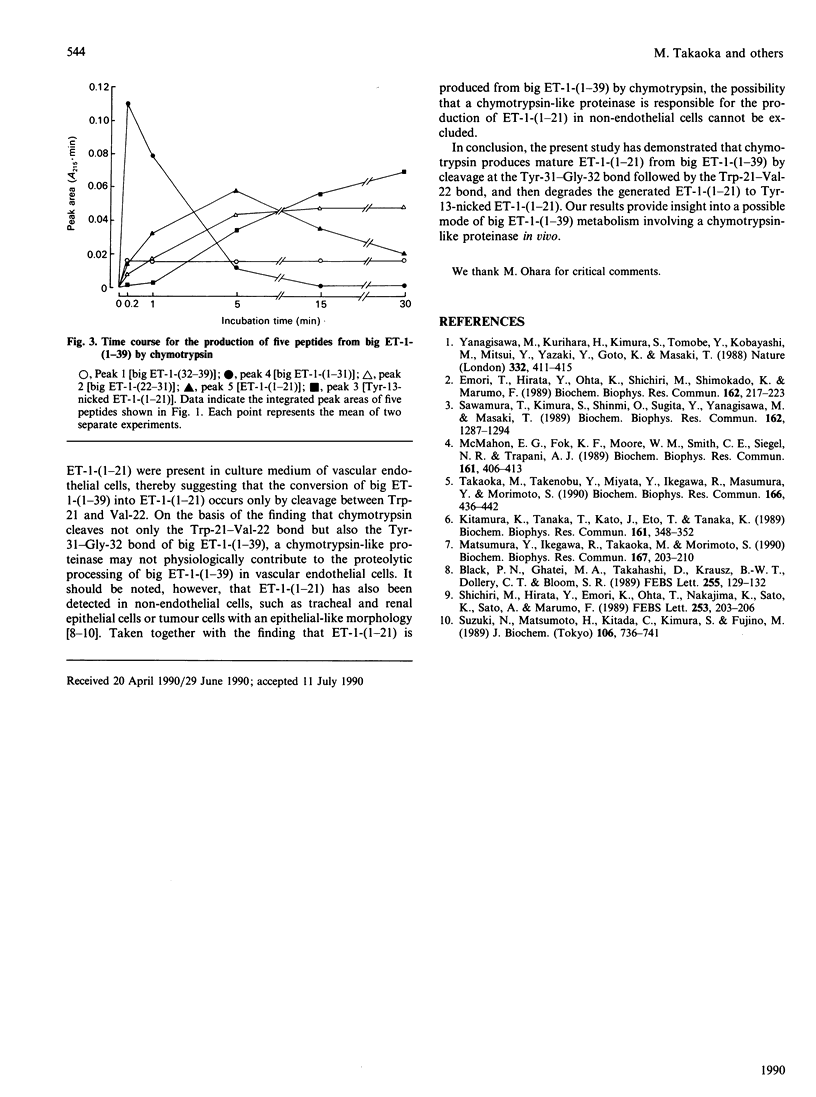
Pig endothelin-1 [ET-1-(1-21)] seems to be produced via proteolytic processing between Trp-21 and Val-22 of an intermediate form consisting of 39 amino acid residues, termed big ET-1-(1-39), by a chymotrypsin-like proteinase. We examined the chymotryptic-cleavage sites of big ET-1-(1-39) by reverse-phase h.p.l.c. and sequence analysis, and found that chymotrypsin cleaved initially the Tyr-31-Gly-32 bond of big ET-1-(1-39), followed by cleavage between Trp-21 and Val-22. Furthermore, chymotrypsin hydrolysed the generated ET-1-(1-21), producing a single major product that had the same amino acid sequence as ET-1-(1-21) with a cleavage between Tyr-13 and Phe-14. The disulphide bridge between Cys-1 and Cys-15 remained intact. These results indicate that the conversion of big ET-1-(1-39) into ET-1-(1-21) catalysed by chymotrypsin requires hydrolysis of the Tyr-31-Gly-32 bond before that of the Trp-21-Val-22 bond, an event followed by cleavage between Tyr-13 and Phe-14 within the loop of ET-1-(1-21). Thus a chymotrypsin-like proteinase might be involved not only in the production but also in the degradation of ET-1-(1-21) in vivo.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black P. N., Ghatei M. A., Takahashi K., Bretherton-Watt D., Krausz T., Dollery C. T., Bloom S. R. Formation of endothelin by cultured airway epithelial cells. FEBS Lett. 1989 Sep 11;255(1):129–132. doi: 10.1016/0014-5793(89)81075-0. [DOI] [PubMed] [Google Scholar]
- Emori T., Hirata Y., Ohta K., Shichiri M., Shimokado K., Marumo F. Concomitant secretion of big endothelin and its C-terminal fragment from human and bovine endothelial cells. Biochem Biophys Res Commun. 1989 Jul 14;162(1):217–223. doi: 10.1016/0006-291x(89)91984-0. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Tanaka T., Kato J., Eto T., Tanaka K. Regional distribution of immunoreactive endothelin in porcine tissue: abundance in inner medulla of kidney. Biochem Biophys Res Commun. 1989 May 30;161(1):348–352. doi: 10.1016/0006-291x(89)91603-3. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Ikegawa R., Takaoka M., Morimoto S. Conversion of porcine big endothelin to endothelin by an extract from the porcine aortic endothelial cells. Biochem Biophys Res Commun. 1990 Feb 28;167(1):203–210. doi: 10.1016/0006-291x(90)91751-d. [DOI] [PubMed] [Google Scholar]
- McMahon E. G., Fok K. F., Moore W. M., Smith C. E., Siegel N. R., Trapani A. J. In vitro and in vivo activity of chymotrypsin-activated big endothelin (porcine 1-40). Biochem Biophys Res Commun. 1989 Jun 15;161(2):406–413. doi: 10.1016/0006-291x(89)92613-2. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Kimura S., Shinmi O., Sugita Y., Yanagisawa M., Masaki T. Analysis of endothelin related peptides in culture supernatant of porcine aortic endothelial cells: evidence for biosynthetic pathway of endothelin-1. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1287–1294. doi: 10.1016/0006-291x(89)90813-9. [DOI] [PubMed] [Google Scholar]
- Shichiri M., Hirata Y., Emori T., Ohta K., Nakajima T., Sato K., Sato A., Marumo F. Secretion of endothelin and related peptides from renal epithelial cell lines. FEBS Lett. 1989 Aug 14;253(1-2):203–206. doi: 10.1016/0014-5793(89)80959-7. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Matsumoto H., Kitada C., Kimura S., Fujino M. Production of endothelin-1 and big-endothelin-1 by tumor cells with epithelial-like morphology. J Biochem. 1989 Nov;106(5):736–741. doi: 10.1093/oxfordjournals.jbchem.a122925. [DOI] [PubMed] [Google Scholar]
- Takaoka M., Takenobu Y., Miyata Y., Ikegawa R., Matsumura Y., Morimoto S. Pepsin, an aspartic protease, converts porcine big endothelin to 21-residue endothelin. Biochem Biophys Res Commun. 1990 Jan 15;166(1):436–442. doi: 10.1016/0006-291x(90)91964-t. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


