Abstract
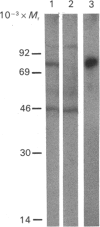
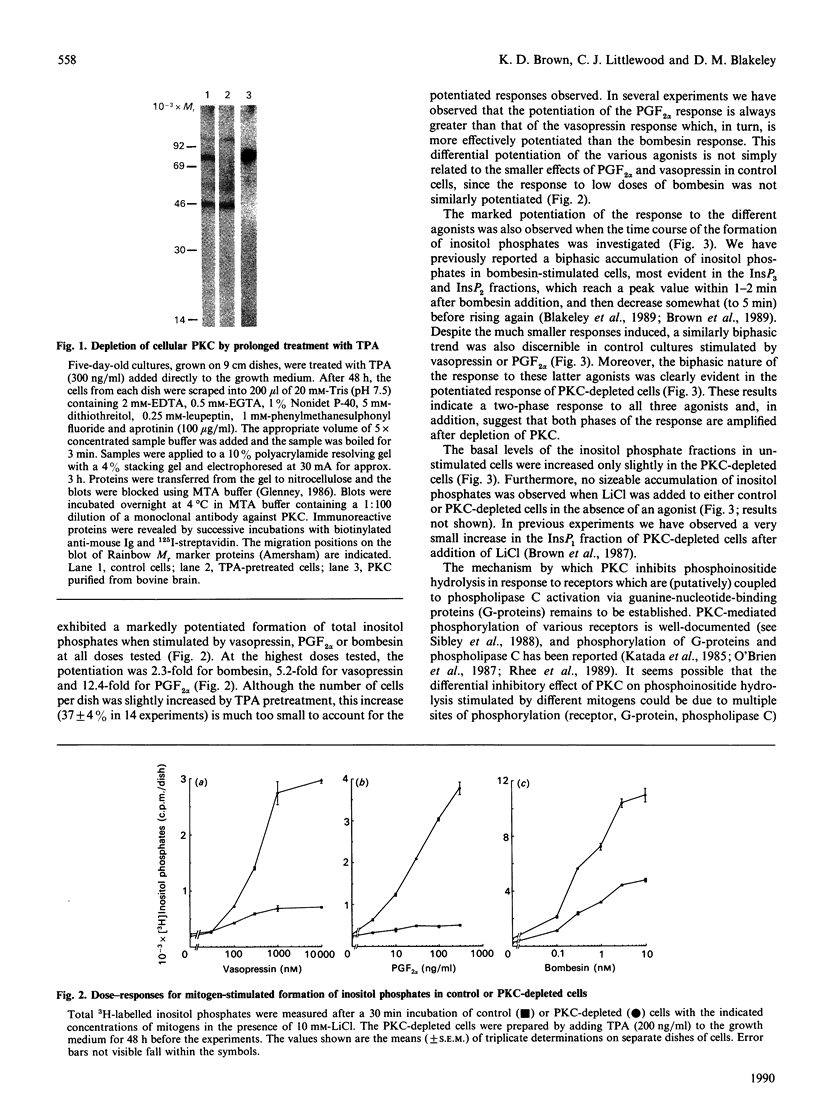
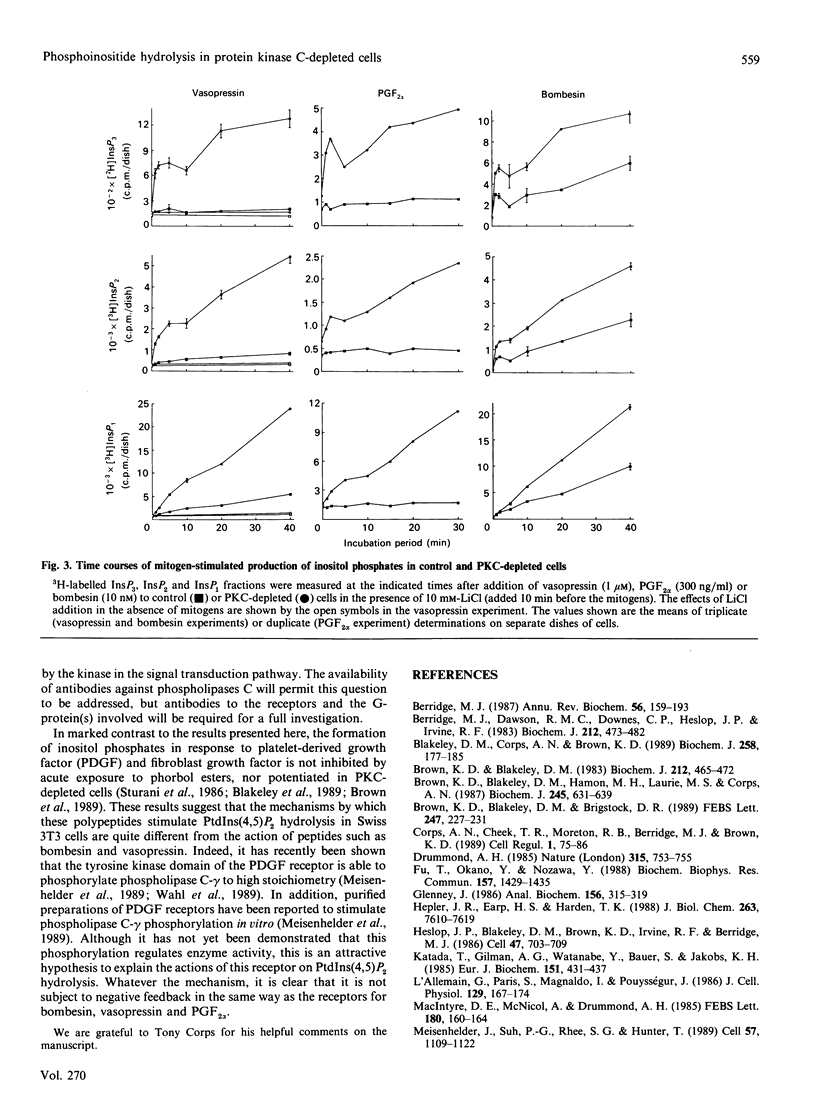
In Swiss 3T3 cells, depletion of protein kinase C (PKC) by prolonged incubation with phorbol esters potentiates the formation of total inositol phosphates in response to bombesin or vasopressin [Blakeley, Corps & Brown (1989) Biochem. J. 258, 177-185]. The characteristics of the accumulation of inositol phosphates in control and PKC-depleted cells stimulated by bombesin, vasopressin or prostaglandin F2 alpha (PGF2 alpha) have now been compared. The potentiation of the PGF2 alpha response was greater than that of the vasopressin response which was, in turn, greater than that of the bombesin response. The time courses of the responses to all three agonists were biphasic, and both phases of the response were amplified in the PKC-depleted cells. These results provide further evidence for the involvement of a PKC-mediated negative-feedback loop regulating phosphoinositide hydrolysis in response to several 3T3 cell mitogens. The differential potentiation of the response to these agonists suggests that PKC might act at multiple sites within the signal transduction pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Blakeley D. M., Corps A. N., Brown K. D. Bombesin and platelet-derived growth factor stimulate formation of inositol phosphates and Ca2+ mobilization in Swiss 3T3 cells by different mechanisms. Biochem J. 1989 Feb 15;258(1):177–185. doi: 10.1042/bj2580177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Brigstock D. R. Stimulation of polyphosphoinositide hydrolysis in Swiss 3T3 cells by recombinant fibroblast growth factors. FEBS Lett. 1989 Apr 24;247(2):227–231. doi: 10.1016/0014-5793(89)81340-7. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M. Inhibition of the binding of 125I-labelled epidermal growth factor to mouse cells by a mitogen in goat mammary secretions. Biochem J. 1983 May 15;212(2):465–472. doi: 10.1042/bj2120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corps A. N., Cheek T. R., Moreton R. B., Berridge M. J., Brown K. D. Single-cell analysis of the mitogen-induced calcium responses of normal and protein kinase C-depleted Swiss 3T3 cells. Cell Regul. 1989 Nov;1(1):75–86. doi: 10.1091/mbc.1.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H. Bidirectional control of cytosolic free calcium by thyrotropin-releasing hormone in pituitary cells. 1985 Jun 27-Jul 3Nature. 315(6022):752–755. doi: 10.1038/315752a0. [DOI] [PubMed] [Google Scholar]
- Fu T., Okano Y., Nozawa Y. Bradykinin-induced generation of inositol 1,4,5-trisphosphate in fibroblasts and neuroblastoma cells: effect of pertussis toxin, extracellular calcium, and down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1429–1435. doi: 10.1016/s0006-291x(88)81035-0. [DOI] [PubMed] [Google Scholar]
- Glenney J. Antibody probing of western blots which have been stained with india ink. Anal Biochem. 1986 Aug 1;156(2):315–319. doi: 10.1016/0003-2697(86)90259-9. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Earp H. S., Harden T. K. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation. Evidence for a role of protein kinase C in agonist-induced desensitization. J Biol Chem. 1988 Jun 5;263(16):7610–7619. [PubMed] [Google Scholar]
- Heslop J. P., Blakeley D. M., Brown K. D., Irvine R. F., Berridge M. J. Effects of bombesin and insulin on inositol (1,4,5)trisphosphate and inositol (1,3,4)trisphosphate formation in Swiss 3T3 cells. Cell. 1986 Dec 5;47(5):703–709. doi: 10.1016/0092-8674(86)90513-1. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Magnaldo I., Pouysségur J. Alpha-thrombin-induced inositol phosphate formation in G0-arrested and cycling hamster lung fibroblasts: evidence for a protein kinase C-mediated desensitization response. J Cell Physiol. 1986 Nov;129(2):167–174. doi: 10.1002/jcp.1041290207. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., McNicol A., Drummond A. H. Tumour-promoting phorbol esters inhibit agonist-induced phosphatidate formation and Ca2+ flux in human platelets. FEBS Lett. 1985 Jan 28;180(2):160–164. doi: 10.1016/0014-5793(85)81063-2. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Monaco M. E., Mufson R. A. Phorbol ester inhibition of the hormone-stimulated phosphoinositide cycle in WRK-1 cells. Biochem J. 1986 May 15;236(1):171–175. doi: 10.1042/bj2360171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Houslay M. D., Milligan G., Siddle K. The insulin receptor tyrosyl kinase phosphorylates holomeric forms of the guanine nucleotide regulatory proteins Gi and Go. FEBS Lett. 1987 Feb 23;212(2):281–288. doi: 10.1016/0014-5793(87)81361-3. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Ochsner M., Whitebread S., De Gasparo M. Down-regulation of protein kinase C potentiates angiotensin II-stimulated polyphosphoinositide hydrolysis in vascular smooth-muscle cells. Biochem J. 1989 Aug 15;262(1):285–291. doi: 10.1042/bj2620285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Dietrich A., Marks F. Molecular cloning of mouse protein kinase C (PKC) cDNA from Swiss 3T3 fibroblasts. Gene. 1988 Dec 30;74(2):465–471. doi: 10.1016/0378-1119(88)90179-5. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Phosphorylation of cell surface receptors: a mechanism for regulating signal transduction pathways. Endocr Rev. 1988 Feb;9(1):38–56. doi: 10.1210/edrv-9-1-38. [DOI] [PubMed] [Google Scholar]
- Sturani E., Vicentini L. M., Zippel R., Toschi L., Pandiella-Alonso A., Comoglio P. M., Meldolesi J. PDGF-induced receptor phosphorylation and phosphoinositide hydrolysis are unaffected by protein kinase C activation in mouse Swiss 3T3 and human skin fibroblasts. Biochem Biophys Res Commun. 1986 May 29;137(1):343–350. doi: 10.1016/0006-291x(86)91216-7. [DOI] [PubMed] [Google Scholar]
- Sugiya H., Obie J. F., Putney J. W., Jr Two modes of regulation of the phospholipase C-linked substance-P receptor in rat parotid acinar cells. Biochem J. 1988 Jul 15;253(2):459–466. doi: 10.1042/bj2530459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., Lapetina E. G. 1,2-Diacylglycerol and phorbol ester inhibit agonist-induced formation of inositol phosphates in human platelets: possible implications for negative feedback regulation of inositol phospholipid hydrolysis. Proc Natl Acad Sci U S A. 1985 May;82(9):2623–2626. doi: 10.1073/pnas.82.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Immunological evidence for two physiological forms of protein kinase C. Mol Cell Biol. 1987 Jan;7(1):85–96. doi: 10.1128/mcb.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]