Abstract
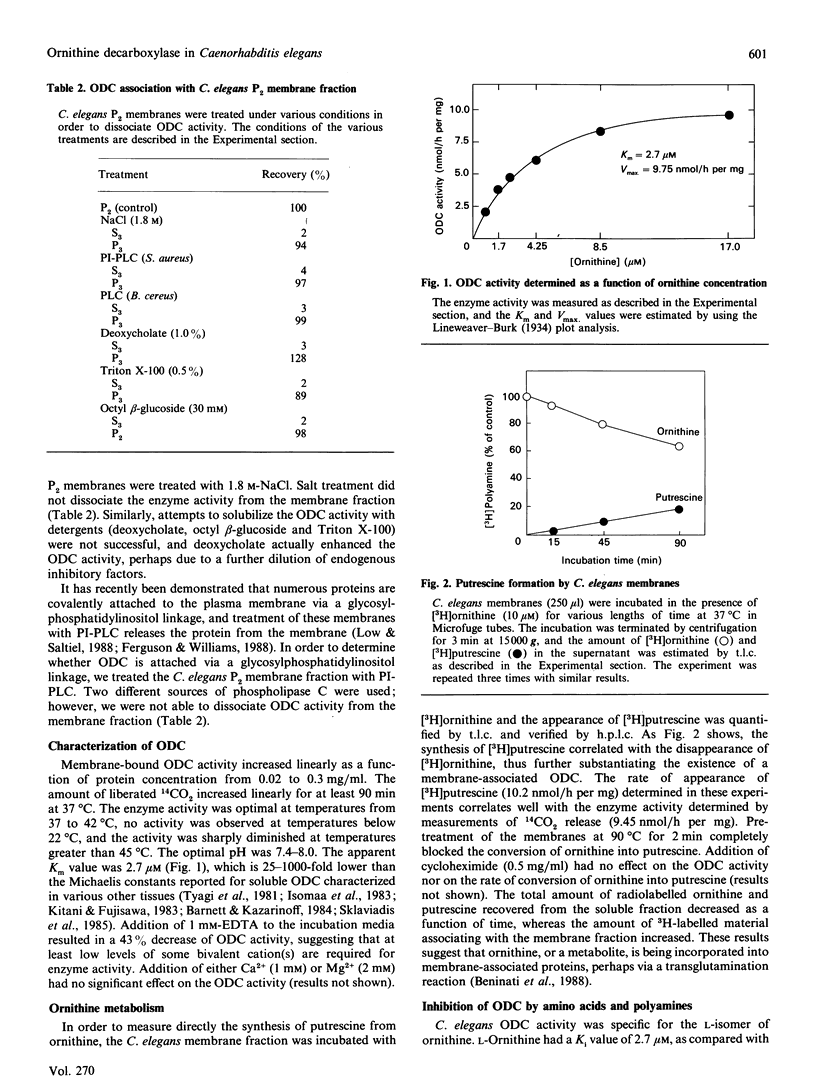
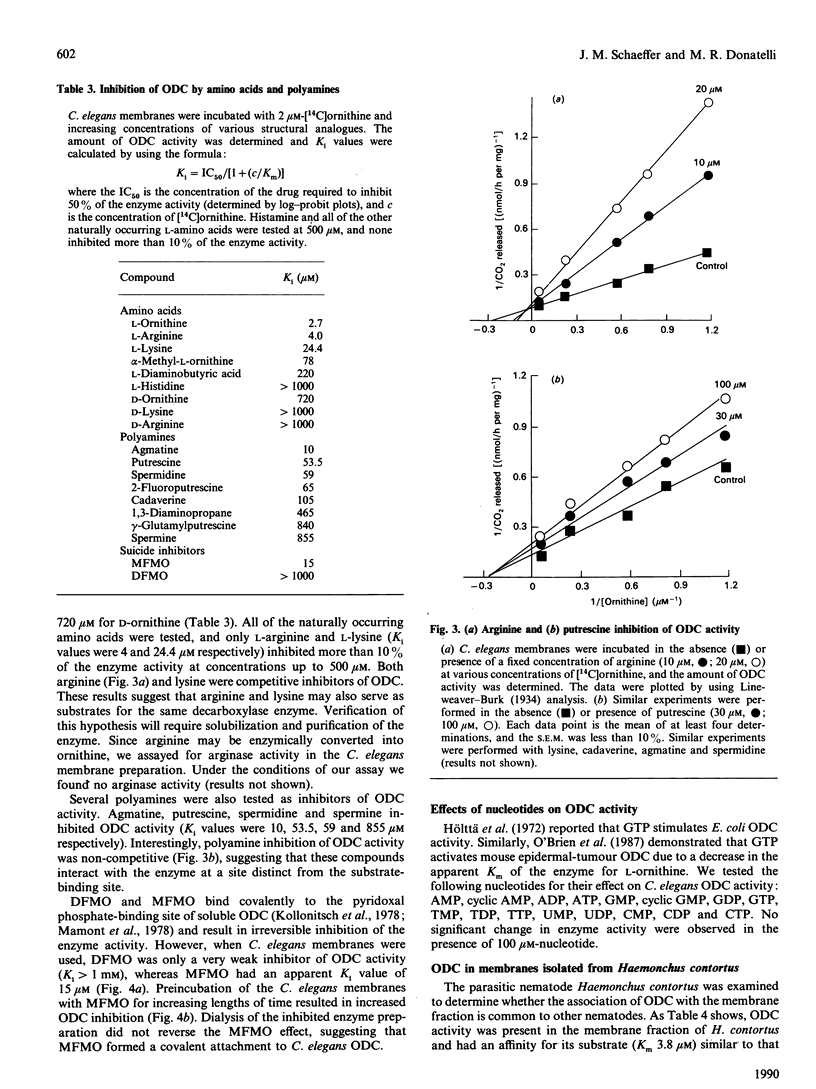
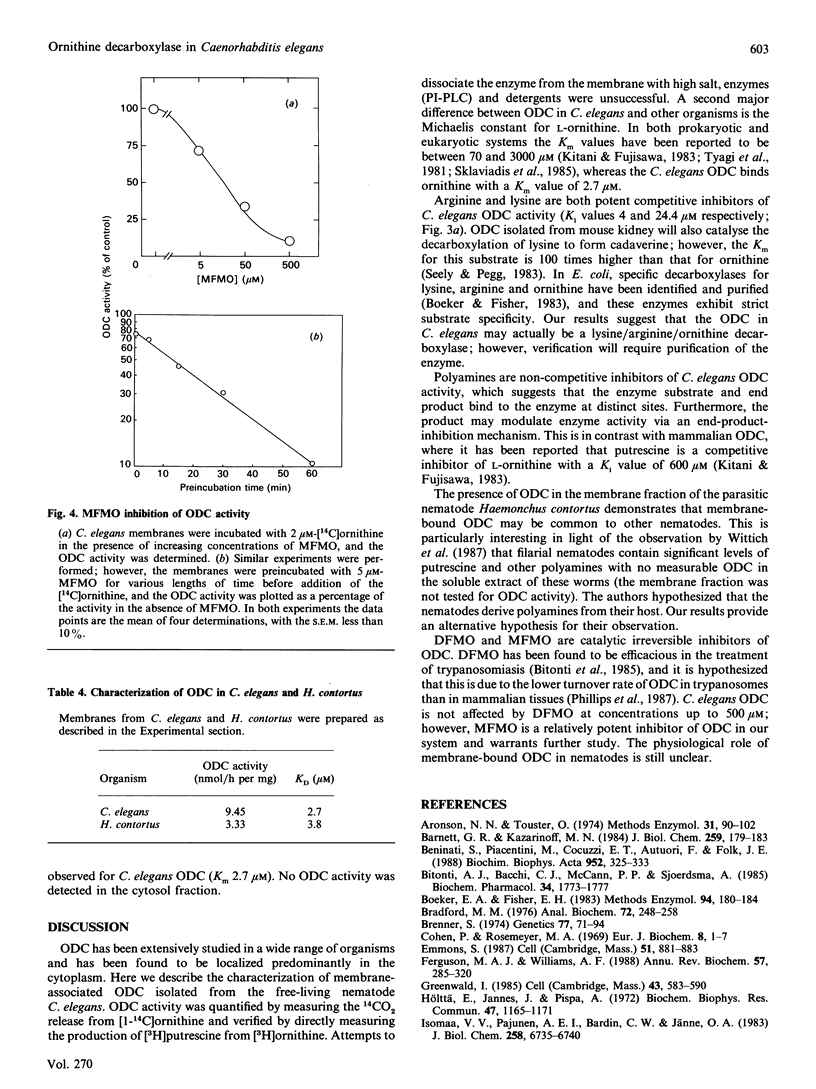
Ornithine decarboxylase has been identified and characterized in the free-living nematode Caenorhabditis elegans. Unlike previously described ornithine decarboxylases, the enzyme activity is membrane-associated and remains in the membrane fraction after treatment with high salt, detergents or phosphatidylinositol-specific phospholipase C. Ornithine has an apparent Km value of 2.7 microM for ornithine decarboxylase. The enzyme is competitively inhibited by arginine and lysine with Ki values of 4.0 and 24.4 microM respectively. None of the other naturally occurring amino acids inhibited more than 10% of the enzyme activity at concentrations up to 1 mM. Agmatine, putrescine, spermidine and spermine inhibit ornithine decarboxylase in a non-competitive manner with Ki values of 10, 53.5, 59 and 855 microM respectively. A similar ornithine decarboxylase activity was also identified in membrane preparations from the parasitic nematode Haemonchus contortus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Barnett G. R., Kazarinoff M. N. Purification and properties of ornithine decarboxylase from Physarum polycephalum. J Biol Chem. 1984 Jan 10;259(1):179–183. [PubMed] [Google Scholar]
- Beninati S., Piacentini M., Cocuzzi E. T., Autuori F., Folk J. E. Covalent incorporation of polyamines as gamma-glutamyl derivatives into CHO cell protein. Biochim Biophys Acta. 1988 Feb 10;952(3):325–333. doi: 10.1016/0167-4838(88)90134-3. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Bacchi C. J., McCann P. P., Sjoerdsma A. Catalytic irreversible inhibition of Trypanosoma brucei brucei ornithine decarboxylase by substrate and product analogs and their effects on murine trypanosomiasis. Biochem Pharmacol. 1985 May 15;34(10):1773–1777. doi: 10.1016/0006-2952(85)90648-3. [DOI] [PubMed] [Google Scholar]
- Boeker E. A., Fischer E. H. Lysine decarboxylase (Escherichia coli B). Methods Enzymol. 1983;94:180–184. doi: 10.1016/s0076-6879(83)94030-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Rosemeyer M. A. Human glucose-6-phosphate dehydrogenase: purification of the erythrocyte enzyme and the influence of ions on its activity. Eur J Biochem. 1969 Mar;8(1):1–7. doi: 10.1111/j.1432-1033.1969.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Emmons S. W. Mechanisms of C. elegans development. Cell. 1987 Dec 24;51(6):881–883. doi: 10.1016/0092-8674(87)90574-5. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Greenwald I. lin-12, a nematode homeotic gene, is homologous to a set of mammalian proteins that includes epidermal growth factor. Cell. 1985 Dec;43(3 Pt 2):583–590. doi: 10.1016/0092-8674(85)90230-2. [DOI] [PubMed] [Google Scholar]
- Isomaa V. V., Pajunen A. E., Bardin C. W., Jänne O. A. Ornithine decarboxylase in mouse kidney. Purification, characterization, and radioimmunological determination of the enzyme protein. J Biol Chem. 1983 Jun 10;258(11):6735–6740. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The nematode Caenorhabditis elegans. Science. 1988 Jun 10;240(4858):1448–1453. doi: 10.1126/science.3287621. [DOI] [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Purification and properties of ornithine decarboxylase from rat liver. J Biol Chem. 1983 Jan 10;258(1):235–239. [PubMed] [Google Scholar]
- Kollonitsch J., Perkins L. M., Patchett A. A., Doldouras G. A., Marburg S., Duggan D. E., Maycock A. L., Aster S. D. Selective inhibitors of biosynthesis of aminergic neurotransmitters. Nature. 1978 Aug 31;274(5674):906–908. doi: 10.1038/274906a0. [DOI] [PubMed] [Google Scholar]
- Laitinen P. H., Huhtinen R. L., Hietala O. A., Pajunen A. E. Ornithine decarboxylase activity in brain regulated by a specific macromolecule, the antizyme. J Neurochem. 1985 Jun;44(6):1885–1891. doi: 10.1111/j.1471-4159.1985.tb07184.x. [DOI] [PubMed] [Google Scholar]
- Low M. G. Phosphatidylinositol-specific phospholipase C from Staphylococcus aureus. Methods Enzymol. 1981;71(Pt 100):741–746. doi: 10.1016/0076-6879(81)71087-5. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G., Hietala O., O'Donnell K., Holmes M. Activation of mouse epidermal tumor ornithine decarboxylase by GTP: evidence for different catalytic forms of the enzyme. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8927–8931. doi: 10.1073/pnas.84.24.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L., Seely J. E., Pegg A. E. Investigation of structure and rate of synthesis of ornithine decarboxylase protein in mouse kidney. Biochemistry. 1984 Jul 31;23(16):3777–3783. doi: 10.1021/bi00311a033. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Coffino P., Wang C. C. Cloning and sequencing of the ornithine decarboxylase gene from Trypanosoma brucei. Implications for enzyme turnover and selective difluoromethylornithine inhibition. J Biol Chem. 1987 Jun 25;262(18):8721–8727. [PubMed] [Google Scholar]
- Porter C. W., Bergeron R. J. Spermidine requirement for cell proliferation in eukaryotic cells: structural specificity and quantitation. Science. 1983 Mar 4;219(4588):1083–1085. doi: 10.1126/science.6823570. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Cavanaugh P. F., Jr, Stolowich N., Ganis B., Kelly E., Bergeron R. J. Biological properties of N4- and N1,N8-spermidine derivatives in cultured L1210 leukemia cells. Cancer Res. 1985 May;45(5):2050–2057. [PubMed] [Google Scholar]
- SCHIMKE R. T. THE IMPORTANCE OF BOTH SYNTHESIS AND DEGRADATION IN THE CONTROL OF ARGINASE LEVELS IN RAT LIVER. J Biol Chem. 1964 Nov;239:3808–3817. [PubMed] [Google Scholar]
- Scott I. G., Pösö H., Akerman K. E., Andersson L. C. Rapid activation of ornithine decarboxylase by mitogenic (but not by nonmitogenic) ligands in human T lymphocytes. Eur J Immunol. 1985 Aug;15(8):783–787. doi: 10.1002/eji.1830150808. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Ornithine decarboxylase (mouse kidney). Methods Enzymol. 1983;94:158–161. doi: 10.1016/s0076-6879(83)94025-9. [DOI] [PubMed] [Google Scholar]
- Seidenfeld J., Block A. L., Komar K. A., Naujokas M. F. Altered cell cycle phase distributions in cultured human carcinoma cells partially depleted of polyamines by treatment with difluoromethylornithine. Cancer Res. 1986 Jan;46(1):47–53. [PubMed] [Google Scholar]
- Sklaviadis T. K., Georgatsos J. G., Kyriakidis D. A. Purification and properties of ornithine decarboxylase from Tetrahymena pyriformis. Biochim Biophys Acta. 1985 Oct 18;831(3):288–296. doi: 10.1016/0167-4838(85)90109-8. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tyagi A. K., Tabor C. W., Tabor H. Ornithine decarboxylase from Saccharomyces cerevisiae. Purification, properties, and regulation of activity. J Biol Chem. 1981 Dec 10;256(23):12156–12163. [PubMed] [Google Scholar]
- Wittich R. M., Kilian H. D., Walter R. D. Polyamine metabolism in filarial worms. Mol Biochem Parasitol. 1987 Jun;24(2):155–162. doi: 10.1016/0166-6851(87)90102-2. [DOI] [PubMed] [Google Scholar]


