Abstract
Steroid groups isolated from many plants are known to play a significant role in various biological systems. Therefore, this research aimed to analyze two novel pregnane steroids, pachylenone A (1) and pachylenone B (2), isolated from Aglaia pachyphylla Miq. The cytotoxicity of the steroids was evaluated against MCF-7 breast cancer cell lines with other known steroid compounds, namely 5α-dihydroprogesterone (3), GSD-8 (4), trans-5α-pregn-l7(20)-en-3,16-dion (5), 20β-hydroxy-5αH-pregnan-3-one (6), 3β-hydroxy-5α-pregnan-20-one (7), aglaiasterol B (8), and 2β,3β-dihydroxypregnan-16-one (9). Meanwhile, structural elucidation was achieved through different spectroscopic methods including one and two-dimensional NMR, as well as mass spectroscopy and quantum chemical calculations (TD-DFT and NMR DP4+ probability). The cytotoxic effects of steroid compounds (1–9) on MCF-7 lines were also examined. The results showed that compound 8 had the strongest activity with an IC50 value of 228 μM, followed by compound 6 (IC50 568,76 μM), and pachylenone A (1) (IC50 768.73 μM). As a recommendation for future research, other activities of these compounds should be evaluated.
Steroid groups isolated from many plants are known to play a significant role in various biological systems.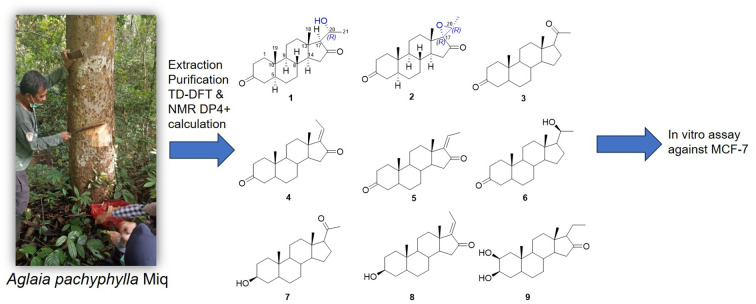
Introduction
Pregnane steroids are secondary metabolites with a significant role in various biological systems used by humans.1 These compounds are typically derived from higher plants and often show a wide range of biological activities due to diverse structures, which have 21 carbons known as the pregnane skeleton (Fig. 1).2 In addition, the syntheses from cholesterol are carried out through complex biosynthetic pathways and are known for structural complexity, which includes multiple rings as well as functional groups and substituents. Steroids are also described as natural hormones found in the human body.3
Fig. 1. Chemical structures of compounds 1–9.
Over 500 unique pregnane steroids have been isolated, showing the extraordinary chemical diversity found in plants. These structural variations contribute to the diverse biological activities, including anti-inflammatory, antibacterial, antifungal, antiviral, and anticancer properties. Previous research reported the cytotoxic potential of pregnane steroids against cancer cell lines.4
The Meliaceae family is well-known for producing a variety of bioactive compounds, including steroids, sesquiterpenoids, and triterpenoids5–7 and is used as traditional medicine.8 Important genera containing biologically active steroids include Aglaia, Trichilia, and Dysoxylum. Aglaia is the most extensive genus, comprising 65 of the 150 species found in Indonesia.9 Several pregnane steroids isolated from the Aglaia genus showed significant cytotoxic activity against cancer cells.10 Previous research reported that pregnane steroids Aglaian A and B were also isolated from A. lawii with a significant value of IC50 against breast cancer cell lines (MCF-7) (50 μM).11
Aglaia pachyphylla Miq., a species within the Aglaia genus, is a tropical plant commonly found in Southeast Asia, including in Indonesia, Malaysia, and the Philippines.8 The cytotoxic potential of pregnane steroids from the species against breast cancer cell lines (MCF-7) has not been extensively analyzed. In the ongoing quest for new bioactive metabolites, a chemical investigation was conducted on Aglaia pachyphylla Miq. obtained from the Kutai Kartanegara, East Kalimantan. Using a variety of methods, two novel pregnane compounds, pachylenone A and B (1 and 2) as well as seven known steroids (3–9) were obtained. The final steps comprised the description of molecule separation, structural analysis, and biological assessment.
Result and discussion
The concentrated ethanolic extract of stem bark of A. pachyphylla Miq. was dissolved in water and extracted respectively with n-hexane, ethyl acetate, and n-butanol. The n-hexane extract was separated using vacuum liquid chromatography (VLC), followed by a combination of column chromatography on normal and reverse phase columns (silica gel G60 and ODS) to gain nine pregnane steroids including two novel pregnane steroids compounds 1 and 2.
Compound 1 was obtained as white amorphous powder, and the molecular formula was established as C21H32O3 based on HRTOF-MS (M + H)+ at m/z 333.2426 (calcd 333.2430), indicating six double bond equivalents (DBEs). The IR spectrum showed absorption bands of hydroxy (3391 cm−1), C–H (2938 cm−1), and two carbonyl stretches (1732 and 1717 cm−1). Moreover, the1H NMR spectrum reported two methyl singlets (δH 0.95 and 1.05), one methyl doublet (δH 1.42, d, 7 Hz), and one hydroxy (δH 4.06, m). The 13C NMR and DEPT spectra also contained 21 carbon resonances, including three methyls at δC 11.6, 13.9, and 23.3, eight methylenes at δC 21.0, 28.8, 31.9, 38.2, 38.5, 39.2, 39.5, and 44.7 ppm, five methines at δC 34.4, 44.7, 50.3, 53.8, and 69.7 ppm, two quaternary carbons at δC 35.9 and 43.1 ppm, one oxymethine at δC 66.5 ppm, as well as two ketones at δC 211.7 and 217.5 ppm.
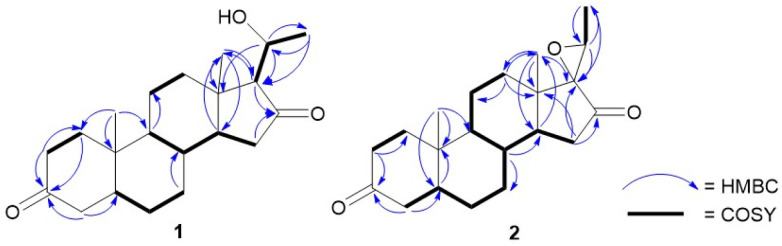
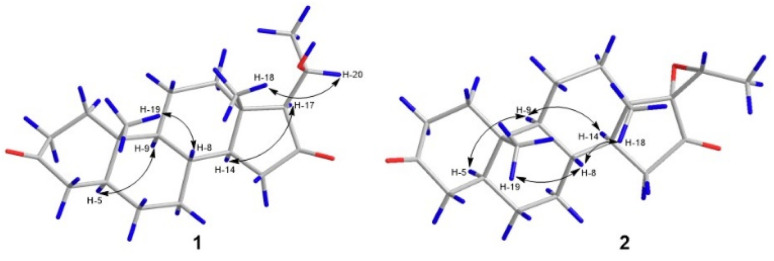
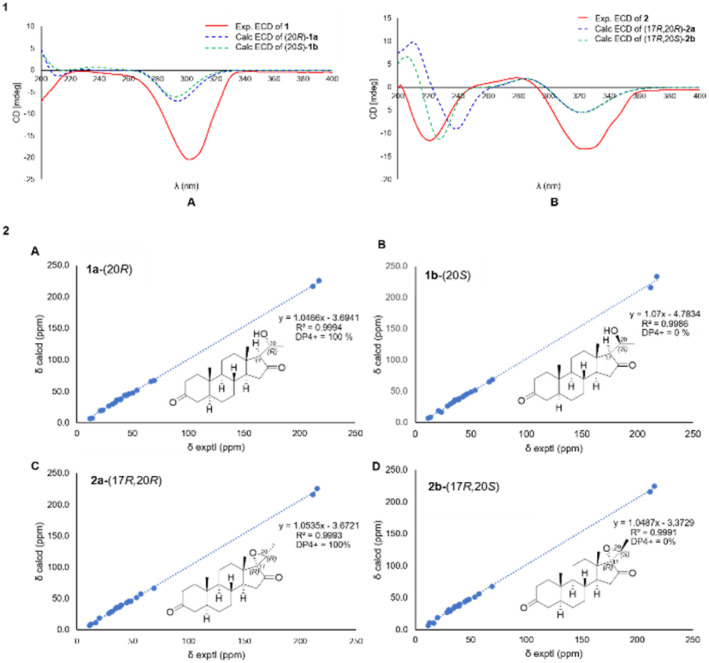
Detailed NMR data analysis suggested that compound 1 was similar to pregnane steroid with 21 carbons.12 The HMBC correlation (Fig. 2) of H-1 (δH 2.37), H-2 (δH 2.04), and H-4 (δH 2.28) to C-3 (δC 211.7) also reported the presence of a ketone group at C-3. Meanwhile, the position of ketone at C-16 was shown by the correlation of H-15 (δC 2.25), and H-17 (δH 1.91) to C-16 (δC 217.5). Correlation between CH3-21 (δH 1.42) to C-20 (δC 66.5), H-20 (δH 4.06) to C-21 (δC 23.3), and C-17 (δC 69.7) indicated the presence of oxymethine and methyl doublet at C-20 and C-21, respectively. In addition, 1H–1H COSY (H-1/H-2, H-5/H-6, H-8/H-9, H-11/H-12, and H-8/H-14/H-15) and H-17/H-20/H-21 correlations confirmed four rings of pregnane skeleton and the position of oxymethine at C-20, respectively. The relative configuration of compound 1 was determined by the NOESY experiment. The correlations (Fig. 3) of H-5 (δH 1.58) and H-14 (δH 1.46) with H-9 (δH 0.93) and H-17 (δH 1.91) showed that H-5, H-9, H-14 and H-7 were in the α orientation. The correlations of CH3-19 (δH 1.05) and CH3-18 (δH 0.95) with H-8 (δH 1.60) and H-20 (δH 4.06) reported that CH3-18, CH3-19, H-8 and H-20 were β-orientation. In addition, hydroxy at C-20 had α-orientation and the configuration was confirmed by ECD experiment (solvent = MeOH) and NMR calculation (DP4+ analysis). In this context, density functional theory (DFT) was stimulated to calculate the 13C NMR chemical shift of two probability C-20 isomers, denoted as (20R)-1a and (20S)-1b. The strong correlation (R2 = 0.9994) was reported by (20R)-1a between the experimental and calculated data based on TD-DFT calculation (Fig. 4). The absolute configuration of compound 1 was shown as 5S, 8R, 9S, 10S, 13S, 14S, 17S, and 20R. Therefore, compound 1 was determined as a novel pregnane steroid and named pachylenone A. The 2D NMR data served as the primary building block for the architecture, as reported in Fig. 1.
Fig. 2. Key HMBC and 1H–1H COSY correlations of pachylenone A and B (1 and 2).
Fig. 3. Key NOESY correlations of pachylenone A and B (1 and 2).
Fig. 4. [1] (A) Calculated and experimental ECD spectra of compound 1, (B) calculated and experimental ECD spectra of compound 2. [2] (A) (1a-20R) and (B) (1b-20S) NMR calculation of compound 1, (C) (2a-17R, 20R) and D (2b-17R, 20S) NMR calculation of compound 2.
Compound 2, colorless needles, had a molecular formula of C21H30O3 based on HRTOF-MS (M + H)+ at m/z 331.2266 (calcd 331.2273), indicating seven DBEs. The IR spectrum showed absorption bands C–H stretch (2924 cm−1), and two carbonyl stretches (1740 and 1710 cm−1). Additionally, the 1H NMR and 13C NMR data were almost similar to compound 1, except for the presence of epoxide at C-17 and C-20. The HMBC correlations (Fig. 2) of H-15 (δH 2.37), H-18 (δH 1.02), H-20 (δH 3.15) and H-21 (δH 1.58) with C-17 (δC 69.0) reported a quarternary carbon at C-17. The correlation between H-21 (δH 1.58) and C-20 (δC 57.2) was confirmed as a methine epoxide at C-20, and the presence was supported by the addition of a double bond equivalent (DBE). The pregnane skeleton and one epoxide ring were shown by the 1H–1H COSY correlation between H-1/H-2, H-4/H-5/H-6/H-7/H-8/H-9/H-11/H-12 and H-8/H-14/H-15 for four rings pregnane and the correlation between H-20 and H-21 for epoxide ring.
The NOESY correlations (Fig. 3) of H-5 (δH 1.58) and H-9 (δH 0.93) with H-9 (δH 0.93) and H-14 (δH 1.71) reported the protons in the α-orientation. Meanwhile, the correlations of H-8 (δH 1.67) with CH3-18 (δH 1.02) and CH3-19 (δH 1.05) showed CH3-18 and CH3-19 in the β-orientation.
The unambiguous configuration for epoxide ring was characterized by the ECD experiment (solvent = MeOH) and calculation of the spectra using TD-DFT method and NMR (DP4+ analysis) (Fig. 4). The closely fitting ECD curve showed that the absolute configuration of compound 2 was determined as 5S, 8R, 9S, 10S, 13S, 14S, 17R, 20R. This was supported by the excellent correlation (R2 = 0.9993) observed between the experimental and calculated data. Therefore, compound 2 had been determined as a novel pregnane-type of steroid and was named pachylenone B.
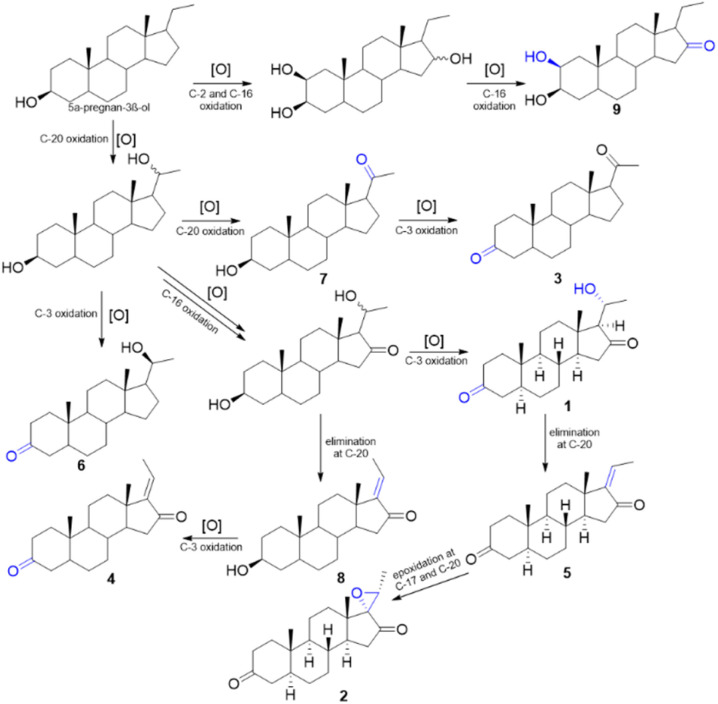
The biogenesis pathway for 1 and 2 was described based on Scheme 1 from 5α-pregnan-3β-ol. This was initiated by oxidation in C-20 and C-16 to suggest a hydroxyy group and ketone, respectively. In addition, the oxidation process at C-3 converted the hydroxy group to ketone, producing compound 1. The elimination process at C-20, which converted hydroxy to an olefinic group, was suggested for compound 5. Subsequently, further epoxidation of the carbon atoms at C-17 and C-20 produced compound 2.
Scheme 1. A plausible biosynthetic pathway of compounds 1–9.
A total of seven known pregnane compounds were obtained from this species including 5α-dihydroprogesterone (3),13 GSD-8 (4),14trans-5α-pregn-l7(20)-en-3.16-dion (5),15 20β-hydroxy-5αH-pregnan-3-one (6),16 3β-hydroxy-5α-pregnan-20-one (7),17 aglaiasterol B (8),18 and 2β,3β-dihydroxypregnan-16-one (9).19
According to Table 2, IC50 values were obtained by assessing the cytotoxic activity of 1–9 against breast cancer cell lines (MCF-7) using the Resazurin (PrestoBlue) assay. Aglaiasterol B (8) possessed the highest activity among pregnane steroids, followed by 20β-hydroxy-5αH-pregnan-3-one (6) (IC50 = 568.76 μM) and pachylenone A (1) (IC50 = 768.73 μM) with an IC50 value of 228 μM. Meanwhile, compounds 1–9 of the pegnane steroids were categorized as inactive against breast cancer cell lines (MCF-7).
Cytotoxic activities of pregnane steroids 1–9 against breast cancer MCF-7 cell lines.
| No. | Compound | IC50 (μM) |
|---|---|---|
| 1 | Pachylenone A (1) | 768.73 |
| 2 | Pachylenone B (2) | >1000 |
| 3 | 5α-Dihydroprogesterone (3) | >1000 |
| 4 | GSD-8 (4) | >1000 |
| 5 | trans-5α-Pregn-l7(20)-en-3,16-dion (5) | >1000 |
| 6 | 20β-Hydroxy-5αH-pregnan-3-one (6) | 568.76 |
| 7 | 3β-Hydroxy-5α-pregnan-20-one (7) | >1000 |
| 8 | Aglaiasterol B (8) | 228 |
| 9 | 2β,3β-Dihydroxypregnan-16-one (9) | >1000 |
| Positive control | (Cisplatin) | 43.18 |
A brief structure–activity relationship (SAR) analysis showed that the cytotoxic activity of aglaiasterol B (8) against MCF-7 cancer cells could be increased directly due to the presence of hydroxyl, carbonyl, and double bond groups at C-3, C-16, and C-17/C-20, respectively. In addition, the presence of the carbonyl substituent (C-16) and the R configuration of the hydroxyl group (C-20) in compound 1 decreased the value of the cytotoxic activity.
Experimental section
General experimental procedures
IR spectra were obtained using a PerkinElmer spectrum-100 FT-IR in the plate of KBR (Waltham, Massachusetts, USA) and Nicolet Summit (Thermo scientific). Meanwhile, optical rotations were measured using an ATAGO AP-300 automatic polarimeter (Saitama, Japan). Electronic circular dichroism spectra were also recorded on a JASCO J-1500 with the detector PM-539. High-resolution mass spectra (HRTOF-MS) were gained on Waters Q-TOF-HRTOFMS-XEVotm mass spectrometer (Milford, MA, USA). Subsequently, NMR spectra were conducted on Bruker ASCEND 700 MHz for 1H, 13C, and 2D-NMR using TMS as the internal standard. Silica gel G60 (Merck, Darmstadt, Germany, 70–230 and 230–400 mesh) and octadecyl silane (ODS, Fuji Sylisia Chemical LTD, Chromatorex® C18 DM1020 M, 100–200 mesh) were also adopted for normal and reverse phase column chromatography (CC). For TLC, Merck pre-coated silica gel 60 F254 plates were performed and spots were detected by spraying with 10% H2SO4 in ethanol before heating.
Plant material
The stem bark of A. pachyphylla Miq. was collected from KHDTK, Kutai Kartanegara, East Kalimantan, Indonesia in December 2020, and was determined at Herbarium Wanariset (WAN), Balikpapan. In addition, a voucher specimen (no. FF11.20) was deposited in the Faculty of Forestry, Mulawarman University.
Extraction and isolation
A total of 4.8 kg of stem bark powder of A. pachyphylla Miq. was macerated in a macerator using ethanol 96% (20 L) 8 × 24 hours at room temperature to obtain the ethanol extract (685 g, 14.2% yield) after the removal of solvent. Furthermore, the concentrated extract was sequentially extracted using n-hexane, EtOAc, and n-BuOH at room temperature. After evaporating with a rotary evaporator, n-hexane (26 g), EtOAc (221 g), and n-BuOH (72 g) extracts were obtained. The n-hexane extract (26 g) was subjected to column chromatography using silica gel, eluted with n-hexane/EtOAc (10 : 0–0 : 10, 10%) and EtOAc/MeOH (10 : 0–8 : 2, 10%) to obtain nine fractions (Fr. A – Fr. I).
Fr. C (4.2 g) was chromatographed using silica gel by a gradient system of n-hexane/EtOAc (10 : 0–8 : 2, 1%) to obtain ten subfractions (Fr. C1–Fr. C10). In addition, Fr C.4 (119 mg) was divided using reverse phase column chromatography (MeOH/water, 9 : 1) to afford compounds 3 (4 mg) and 4 (7.5 mg). Recrystallization process with MeOH was also used to purify Fr C6 and C7 in obtaining compounds 5 (14 mg) and 6 (55 mg). Fr C8 was separated on RP-18 silica gel CC (MeOH/water, 9 : 1) to provide compound 7 (7 mg), while Fr. C9 (150 mg) obtained 1 (7 mg) and 2 (8.5 mg). A total of five subfractions (Fr E1–E5) was reported by subjecting Fr E (3.3 g) to silica gel CC eluted with n-hexane/EtOAc (8 : 2–6 : 4, 2%). An isocratic system n-hexane/DCM/EtOAc (6 : 2 : 2) was used to purify Fr E3 (803 mg) in obtaining compound 8 (8.3 mg). Fr F (2.6 g) was separated using a gradient system of normal phase column chromatography, eluted with n-hexane/EtOAc (7 : 3–1 : 1, 2%) to obtain seven subfractions (Fr F1–F7). Subsequently, Fr F4 (469 mg) was treated to obtain four subfractions (Fr F4a–F4d). Compound 9 (5.2 mg) was reported by purifying Fr F4b on RP-18 silica gel CC (MeOH/water, 7 : 3).
Pachylenone A (1): white amorphous powder, [α]26D -95.2 (c 0.4, CHCl3), IR (KBr) vmax 3391, 2938, 2854, 1732, 1717 cm−1, HRTOF-MS m/z 333.2426 [M+H]+ (calc. for C21H33O3, 333.2430), 1H-NMR (CDCl3, 700 MHz) and 13C-NMR (CDCl3, 175 MHz), as shown in Table 1.
NMR data (700 MHz for 1H and 175 MHz for 13C in CDCl3) for 1 and 2.
| Position | 1 | 2 | ||
|---|---|---|---|---|
| 13C NMR | 1H NMR | 13C NMR | 1H NMR | |
| δ C (mult.) | δ H (integral, mult, J Hz) | δ C (mult.) | δ H (integral, mult, J Hz) | |
| 1 | 38.2 (t) | 2.04 (1H, m) | 38.1 (t) | 2.03 (1H, m) |
| 1.37 (1H, m) | 1.46 (1H, m) | |||
| 2 | 38.5 (t) | 2.37 (1H, m) | 38.0 (t) | 2.35 (1H, m) |
| 2.33 (1H, m) | 2.03 (1H, m) | |||
| 3 | 211.7 (s) | 211.4 (s) | ||
| 4 | 44.7 (t) | 2.11 (1H, m) | 44.5 (t) | 2.27 (1H, m) |
| 2.28 (1H, m) | 2.11 (1H, m) | |||
| 5 | 46.7 (d) | 1.58 (1H, m) | 46.5 (d) | 1.58 (1H, m, overlap) |
| 6 | 28.8 (t) | 1.38 (2H, m) | 28.6 (t) | 1.37 (2H, m) |
| 7 | 31.9 (t) | 1.66 (1H, m) | 31.4 (t) | 1.70 (1H, m) |
| 1.01 (1H, dd, 14; 7) | 1.03 (1H, m) | |||
| 8 | 34.4 (d) | 1.60 (1H, m) | 34.6 (d) | 1.67 (1H, m) |
| 9 | 53.8 (d) | 0.93 (1H, m) | 53.4 (d) | 0.93 (1H, m) |
| 10 | 35.9 (s) | 35.9 (s) | ||
| 11 | 21.0 (t) | 1.65 (1H, m) | 20.1 (t) | 1.71 (1H, m) |
| 1.49 (1H, m) | 1.46 (1H, m) | |||
| 12 | 39.2 (t) | 1.86 (2H, m) | 30.2 (t) | 1.46 (1H, m) |
| 1.28 (1H, dd, 14; 7) | ||||
| 13 | 43.1 (s) | 39.8 (s) | ||
| 14 | 50.3 (d) | 1.46 (1H, m) | 48.5 (d) | 1.71 (1H, m, overlap) |
| 15 | 39.5 (t) | 2.25 (1H, m) | 39.7 (t) | 2.37 (1H, d, 7) |
| 1.48 (1H, m) | 2.15 (1H, d, 7) | |||
| 16 | 217.5 (s) | 215.2 (s) | ||
| 17 | 69.7 (d) | 1.91 (1H, d, 7) | 69.0 (s) | |
| 18 | 13.9 (q) | 0.95 (3H, s) | 16.8 (q) | 1.02 (3H, s) |
| 19 | 11.6 (q) | 1.05 (3H, s) | 11.5 (q) | 1.05 (3H, s) |
| 20 | 66.5 (d) | 4.06 (1H, m) | 57.2 (d) | 3.15 (1H, q, 7) |
| 21 | 23.3 (q) | 1.42 (3H, d, 7) | 12.7 (q) | 1.58 (3H, d, 7) |
Pachylenone B (2): colorless needles, [α]26D -55.3 (c 0.4, CHCl3), IR vmax 2924, 1740, 1710 cm−1, HRTOF-MS m/z 331.2266 [M + H]+ (calc. for C21H31O3, 331.2273); 1H-NMR (CDCl3, 700 MHz) and 13C-NMR (CDCl3, 175 MHz), as shown in Table 1.
Methods for ECD and NMR calculation
Conformational distribution searches were performed using Spartan 14.0 (Wavefunction Inc., Irvine, CA, USA) software with a Boltzman distribution of 2% using the Merck molecular force field (MMFF). The optimization of conformers was performed using DFT method at B3LYP/6-31g(d) level on the Gaussian 09 program. In addition, the optimized conformer was subjected to TD-DFT calculation at B3LYP/6-311g(d,p) level, nstates = 20, and a polarizable continuum model (IEFPCM, solvent: MeOH). The spectra were generated to SpecDis 1.71 with sigma = 0.25 eV and the Gauge-Independent Atomic Orbital (GIAO) method at MPW1PW91/6-311+g(d,p) (IEFPCM, solvent: chloroform) was used for NMR calculation.20
Cytotoxic bioassay of compounds 1–9
The PrestoBlue assay, as documented by Mulyani and Nurlelasari (2024)21,22 was used to evaluate the cytotoxic potential. Compounds 1–9 were evaluated against breast cancer cell lines (MCF-7) (ATCC HTB-22) cultivated in RPMI medium. These cells were supplemented with 10% Fetal Bovine Serum (FBS) and 1 μL mL−1 penicillin-type antibiotic (Sigma Aldrich P4333). Additionally, incubation was carried out at 37 °C in a humidified atmosphere with 5% CO2. Cell seeding was performed in 96-well plates at a density of 1.7 × 104 and the introduction of compounds 1–9 of varying concentrations (2.34, 4.69, 9.38, 18.75, 37.50, 75.00, 150, 300 μg mL−1) into the wells occurred separately. The stock drug solutions were made with cisplatin (positive control) as much as 1 mg was dissolved in 1 mL phosphate buffered saline (PBS, Gibco 18912-014). After 48 hours, an assessment of cell viability was performed by quantifying the conversion of resazurin to resorufin, emitting a pink fluorescent signal denoting viable cells. Meanwhile, the analysis of results unfolded through a multimode reader at 570 nm, and the IC50 values were deduced from graphs contrasting the percentage of viable cells to DMSO 2% in media 1 mL as a negative control. The values showed the concentration requisite to impede cell growth by 50%. Preliminary SAR was demonstrated by the substituents at C-3, 5, 6, 7, 15, 16, 17, and 21, which was affecting cytotoxic activity.23
Conclusions
In conclusion, this research was conducted to successfully examine two novel pregnane steroids, pachylenone A (1) and pachylenone B (2), with seven compounds isolated from Aglaia pachyphylla Miq. The chemical activities of the steroids were enhanced and the interest in chemical synthesis was promoted to recreate the potency and selectivity of natural compounds. Moreover, other bioactivities and mechanisms of action for compounds (1–9) in killing cancer cells were evaluated.
Data availability
Data supporting this study are included within the article and ESI.†
Author contributions
All authors contributed to the writing of the manuscript and have approved the final version.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors are grateful to the Indonesian Ministry of Research, Technology, and Higher Education for Grant of Pendidikan Magister menuju Doktor untuk Sarjana Unggul (PMDSU) (3166/UN6.3.1/PT.00/2024; 6 Mei 2024) Indonesia, Academic Leadership Grant (ALG) (1433/UN6.3.1/PT.00/2024; 18 Maret 2024) by Desi Harneti, and the Directorate of Research and Community Engagement Universitas Padjadjaran, for providing support during the research. In addition, the authors are thankful to Universitas Padjadjaran for providing the required facilities.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04727c
References
- Li J. F. et al., Structurally diverse steroids with nitric oxide inhibitory activities from Aglaia lawii leaves. Phytochemistry. 2021;183:112651. doi: 10.1016/j.phytochem.2020.112651. [DOI] [PubMed] [Google Scholar]
- Zhu G. L. et al., C21, C22 pregnane glycosides and cytotoxic C27 spriostanol steroids from Asparagus cochinchinesis. Steroids. 2021;172:108874. doi: 10.1016/j.steroids.2021.108874. [DOI] [PubMed] [Google Scholar]
- Zhao M. et al., Science of the Total Environment Reproductive and transgenerational toxicity of bisphenol S exposure in pregnant rats : Insights into hormonal imbalance and steroid biosynthesis pathway disruption. Sci. Total Environ. 2024;927:172379. doi: 10.1016/j.scitotenv.2024.172379. [DOI] [PubMed] [Google Scholar]
- Gomes A. R. Pires A. S. Roleira F. M. F. Tavares-da-Silva E. J. The Structural Diversity and Biological Activity of Steroid Oximes. Molecules. 2023;28(4):1690. doi: 10.3390/molecules28041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayanti T. Sinaga S. E. Supratman U. Phytochemistry and biological activity of Lansium domesticum Corr. species: a review. J. Pharm. Pharmacol. 2022:1568–1587. doi: 10.1093/jpp/rgac057. [DOI] [PubMed] [Google Scholar]
- Sinaga S. E. Abdullah F. F. Supratman U. Mayanti T. Maharani R. Isolation and Structure Determination of Stigmaterol from the Stem Bark of Lansium domesticum Corr. Cv. Kokossan. Chim. Nat. Acta. 2022;10(3):106–111. [Google Scholar]
- Safriansyah W. Sinaga S. E. Supratman U. Harneti D. Phytochemistry and Biological Activities of Guarea Genus (Meliaceae) Molecules. 2022;27(24):8758. doi: 10.3390/molecules27248758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safriansyah W. et al., Sesquiterpenoids from the stem bark of Aglaia pachyphylla Miq (Meliaceae) and cytotoxic activity against MCF-7 Cancer Cell Line. J. Kim. Valensi. 2023;9(2):300–305. [Google Scholar]
- Harneti D. Supratman U. Phytochemistry and biological activities of Aglaia species. Phytochemistry. 2021;181:112540. doi: 10.1016/j.phytochem.2020.112540. [DOI] [PubMed] [Google Scholar]
- Mouth H G. Teufel R. Steroids from the Meliaceae family and their biological activities. Phytochemistry. 2024;221:114039. doi: 10.1016/j.phytochem.2024.114039. [DOI] [PubMed] [Google Scholar]
- Dong J. Liu H. Wang H. Lou H. Pan W. Li J. Bioactivities of Steroids and Sesquiterpenes from the Branches and Leaves of Aglaia lawii. Molecules. 2023;29(1):39. doi: 10.3390/molecules29010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. D. Wu S. H. Ma Y. B. Wu D. G. Components of Cipadessa baccifera. Phytochemistry. 2000;55(8):867–872. doi: 10.1016/s0031-9422(00)00247-8. [DOI] [PubMed] [Google Scholar]
- Zeng C. Liu X. Sun C. Xu X. Peng L. High Enantioselective and Large-Scale Production of Ursodeoxycholic Acid by Combination of Pd- and Hydroxysteroid Dehydrogenase-Catalyzed Hydrogenation. Org. Process Res. Dev. 2024;28(4):1260–1268. [Google Scholar]
- Kohyama A. et al., Synthesis of guggulsterone derivatives as potential anti-austerity agents against PANC-1 human pancreatic cancer cells. Bioorg. Med. Chem. Lett. 2020;30(7):126964. doi: 10.1016/j.bmcl.2020.126964. [DOI] [PubMed] [Google Scholar]
- von Gunter A. Schreiber K. Konfiguration und Umsetzungen von 20-stereoisomeren 20-Chlor- 16B-hydroxy-pregnanen. Heterolytische Fragmentierung zu 16.17-Secosteroidaldehyden. Liebigs Ann. Chem. 1967;202:191–202. doi: 10.1002/jlac.19677090120. [DOI] [PubMed] [Google Scholar]
- Monsalve L. N. Machado M. Y. Ghini A. A. Baldessari A. An efficient enzymatic preparation of 20-pregnane succinates : chemoenzymatic synthesis of 20 b -hemisuccinyloxy- 5 a H -pregnan-3-one. Tetrahedron. 2008;64:1721–1730. [Google Scholar]
- Macnevin C. J. Atif F. Sayeed I. Stein D. G. Liotta D. C. Development and Screening of Water-Soluble Analogues of Progesterone and Allopregnanolone in Models of Brain Injury. J. Med. Chem. 2009;52:6012–6023. doi: 10.1021/jm900712n. [DOI] [PubMed] [Google Scholar]
- Zhang F. Zhu Y. Li Q. Cen J. Four New Pregnane Steroids from Aglaia abbreviata and Their Cytotoxic Activities. Helv. Chim. Acta. 2016;99:73–77. [Google Scholar]
- Pupo M. T. Vieira C. Fernandes J. B. Da Silva M. F. D. G. Fo E. R. Androstane and pregnane 2p, 19-hemiketal steroids from Trichilia claussenii. Phytochemistry. 1997;45(7):1495–1500. [Google Scholar]
- Grimblat N. Zanardi M. M. Sarotti A. M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015;80(24):12526–12534. doi: 10.1021/acs.joc.5b02396. [DOI] [PubMed] [Google Scholar]
- Mulyani Y. et al., Arohynapene A Produced by Penicillium steckii JB-NW-2-1 Isolated from Avicennia marina (Forssk.) Vierh and Its Cytotoxic Activities. Indones. J. Chem. 2024;24(2):560. [Google Scholar]
- Nurlelasari N. et al., A Novel Limonoid from the Seeds of Chisocheton macrophyllus. Rec. Nat. Prod. 2024;18(3):347–351. [Google Scholar]
- Huang Y. Li G. Hong C. Zheng X. Yu H. Z Y. Potential of Steroidal Alkaloids in Cancer: Perspective Insight Into Structure–Activity Relationships. Front. Oncol. 2021;11:733369. doi: 10.3389/fonc.2021.733369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this study are included within the article and ESI.†