Abstract
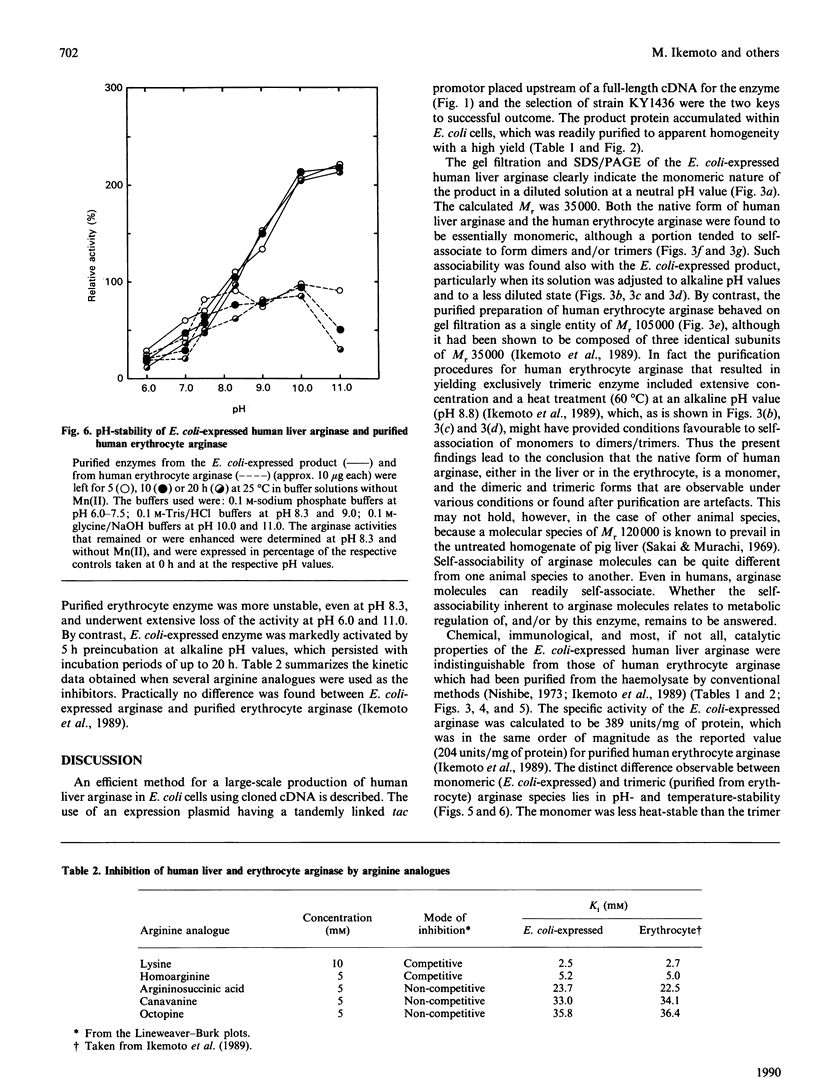
Arginase is an enzyme that catalyses the hydrolysis of arginine to urea and ornithine. It is abundantly present in the liver of ureotelic animals (i.e. those whose excretion is characterized by the excretion of uric acid as the chief end-product of nitrogen metabolism), but its purification has hitherto not been simple, and the yield not high. Starting with a partially truncated cDNA for human liver arginase recently made available, we constructed an expression plasmid that had tandemly linked tac promotors placed upstream of a full-length cDNA. By selecting Escherichia coli strain KY1436 as the host micro-organism, we established an efficient system for the production of human liver arginase protein. Chromatographies on CM-Sephadex G-150, DEAE-cellulose and Sephadex G-150, followed by preparative agar-gel electrophoresis, yielded 10 mg of apparently homogeneous enzyme protein from 1 g (wet wt.) of E. coli cells. E. coli-expressed human liver arginase had chemical, immunological and most catalytic properties indistinguishable from those of purified human erythrocyte arginase. However, E. coli-expressed arginase was a monomer of Mr 35,000, whereas the purified erythrocyte arginase was trimer of Mr 105,000. They differed also in pH- and temperature-stabilities. Gel-filtration experiments with these two purified arginases under various conditions, as well as with unfractionated human liver and erythrocyte cytosol preparations, indicated that the native form of human arginase should be of Mr 35,000, and that the trimeric appearance of human erythrocyte arginase after purification was an artifact of the purification procedures. It was thus concluded that, in Nature, the liver and erythrocyte arginases are identical proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascur L., Cabello J., Véliz M., González A. Molecular forms of human-liver arginase. Biochim Biophys Acta. 1966 Oct 17;128(1):149–154. doi: 10.1016/0926-6593(66)90151-2. [DOI] [PubMed] [Google Scholar]
- Bernar J., Hanson R. A., Kern R., Phoenix B., Shaw K. N., Cederbaum S. D. Arginase deficiency in a 12-year-old boy with mild impairment of intellectual function. J Pediatr. 1986 Mar;108(3):432–435. doi: 10.1016/s0022-3476(86)80891-5. [DOI] [PubMed] [Google Scholar]
- Berüter J., Colombo J. P., Bachmann C. Purification and properties of arginase from human liver and erythrocytes. Biochem J. 1978 Nov 1;175(2):449–454. doi: 10.1042/bj1750449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Brusdeilins M., Kühner R., Schumacher K. Purification, affinity to anti-human arginase immunoglobulin-Sepharose 4B and subunit molecular weights of mammalian arginases. Biochim Biophys Acta. 1985 May 29;840(1):79–90. doi: 10.1016/0304-4165(85)90164-3. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Cederbaum S. D., Shaw K. N., Valente M. Hyperargininemia. J Pediatr. 1977 Apr;90(4):569–573. doi: 10.1016/s0022-3476(77)80368-5. [DOI] [PubMed] [Google Scholar]
- GREENBERG D. M., BAGOT A. E., ROHOLT O. A., Jr Liver arginase. III. Properties of highly purified arginase. Arch Biochem Biophys. 1956 Jun;62(2):446–453. doi: 10.1016/0003-9861(56)90143-6. [DOI] [PubMed] [Google Scholar]
- Grazi E., Magri E. Molecular characteristics of chicken liver arginase. Biochem J. 1972 Feb;126(3):667–674. doi: 10.1042/bj1260667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi Y., Takiguchi M., Amaya Y., Kawamoto S., Matsuda I., Mori M. Molecular cloning and nucleotide sequence of cDNA for human liver arginase. Proc Natl Acad Sci U S A. 1987 Jan;84(2):412–415. doi: 10.1073/pnas.84.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harell D., Sokolovsky M. Beef-liver arginase. Isolation and molecular properties. Eur J Biochem. 1972 Jan 31;25(1):102–108. doi: 10.1111/j.1432-1033.1972.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Yumoto N., Sakihama T., Murachi T. Evidence for heterodimeric nature of calpain molecules as studied by cross-linking modification. Biochem Int. 1985 Feb;10(2):187–193. [PubMed] [Google Scholar]
- Hirsch-Kolb H., Greenberg D. M. Molecular characteristics of rat liver arginase. J Biol Chem. 1968 Dec 10;243(23):6123–6129. [PubMed] [Google Scholar]
- Ikemoto M., Tabata M., Murachi T., Totani M. Purification and properties of human erythrocyte arginase. Ann Clin Biochem. 1989 Nov;26(Pt 6):547–553. doi: 10.1177/000456328902600616. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Imagawa M., Hashida S., Yoshitake S., Hamaguchi Y., Ueno T. Enzyme-labeling of antibodies and their fragments for enzyme immunoassay and immunohistochemical staining. J Immunoassay. 1983;4(3):209–327. doi: 10.1080/15321818308057011. [DOI] [PubMed] [Google Scholar]
- Joyce B. G., Read G. F., Fahmy D. R. A specific enzymeimmunoassay for progesterone in human plasma. Steroids. 1977 Jun;29(6):761–770. doi: 10.1016/0039-128x(77)90120-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishibe H. Isolation and characterizaiton of arginase in human erythrocyte. Physiol Chem Phys. 1973;5(6):453–462. [PubMed] [Google Scholar]
- Ozer N. A new enzyme-coupled spectrophotometric method for the determination of arginase activity. Biochem Med. 1985 Jun;33(3):367–371. doi: 10.1016/0006-2944(85)90012-2. [DOI] [PubMed] [Google Scholar]
- Spector E. B., Rice S. C., Cederbaum S. D. Immunologic studies of arginase in tissues of normal human adult and arginase-deficient patients. Pediatr Res. 1983 Dec;17(12):941–944. doi: 10.1203/00006450-198312000-00003. [DOI] [PubMed] [Google Scholar]
- Stark M. J. Multicopy expression vectors carrying the lac repressor gene for regulated high-level expression of genes in Escherichia coli. Gene. 1987;51(2-3):255–267. doi: 10.1016/0378-1119(87)90314-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yano R., Imai M., Yura T. The use of operon fusions in studies of the heat-shock response: effects of altered sigma 32 on heat-shock promoter function in Escherichia coli. Mol Gen Genet. 1987 Apr;207(1):24–28. doi: 10.1007/BF00331486. [DOI] [PubMed] [Google Scholar]