Abstract
Two new genera, 17 new species, two epitypes, and six interesting new host and / or geographical records are introduced in this study. New genera include: Cadophorella (based on Cadophorella faginea) and Neosatchmopsis (based on Neosatchmopsis ogrovei). New species include: Alternaria halotolerans (from hypersaline sea water, Qatar), Amylostereum stillwellii (from mycangia of Sirex areolatus, USA), Angiopsora anthurii (on leaves of Anthurium andraeanum, Brazil), Anthracocystis zeae-maydis (from pre-stored Zea mays, South Africa), Bisifusarium solicola (from soil, South Africa), Cadophorella faginea (from dead capsule of Fagus sylvatica, Germany), Devriesia mallochii (from house dust, Canada), Fusarium kirstenboschense (from soil, South Africa), Macroconia podocarpi (on ascomata of ascomycete on twigs of Podocarpus falcatus, South Africa), Neosatchmopsis ogrovei (on Eucalyptus leaf litter, Spain), Ophiocordyceps kuchinaraiensis (on Coleoptera larva, Thailand), Penicillium cederbergense (from soil, South Africa), Penicillium pascuigraminis (from pasture mulch, South Africa), Penicillium viridipigmentum (from soil, South Africa), Pleurotheciella acericola (on stem, bark of living tree of Acer sp., Germany), Protocreopsis physciae (on Physcia caesia, Netherlands), and Talaromyces podocarpi (from soil, South Africa).
Citation: Visagie CM, Yilmaz N, Allison JD, Barreto RW, Boekhout T, Boers J, Delgado MA, Dewing C, Fitza KNE, Furtado ECA, Gaya E, Hill R, Hobden A, Hu DM, Hülsewig T, Khonsanit A, Kolecka A, Luangsa-ard JJ, Mthembu A, Pereira CM, Price J-L, Pringle A, Qikani N, Sandoval-Denis M, Schumacher RK, Slippers B, Tennakoon DS, Thanakitpipattana D, van Vuuren NI, Groenewald JZ, Crous PW (2024). New and Interesting Fungi. 7. Fungal Systematics and Evolution 13: 441–494. doi: 10.3114/fuse.2024.13.12
Keywords: biodiversity, ITS barcodes multi-gene phylogeny new taxa systematics typification
INTRODUCTION
Most of the world’s fungi remain unknown. Mycologists have long referred to an estimate of 1.5 million species calculated based on a ratio of six fungi to one plant species ( Hawksworth 1991). A more recent estimate is 2.2 to 3.8 million species of fungi ( Hawksworth & Lucking 2017), but some suggest this figure could be as high as 5.1 million ( Blackwell 2011) or even higher ( Locey & Lennon 2016). Currently, the “best estimate” is revised to be 2.5 million species ( Niskanen et al. 2023). The higher estimates are largely due to the development of technologies that have transformed how we define and identify species. For example, DNA sequencing efforts and the subsequent application of a phylogenetic species concept for most fungal groups has led to the recognition of cryptic species and a more progressive approach to the description of new species. Another influence is the rapid rise of Next Generation Sequencing technologies, which enabled high throughput metabarcoding studies and highlight ecosystems with hidden undescribed species diversity ( Nilsson et al. 2016, Nilsson et al. 2019). Currently, just over 150 000 fungal species have been described, which is about 6 % considering the lower end of an estimate of 2.5 million ( Antonelli et al. 2020, Index Fungorum Partnership 2024).
South Africa is recognised as one of the most biodiverse countries in the world ( Myers et al. 2000) and is home to three of the world’s 36 biodiversity hotspots, including the Cape Floristic Region, the Succulent Karoo and Maputaland-Pondoland-Albany (Conservation International: https://www.cepf.net/). Mucina & Rutherford (2006) listed 435 vegetation types for the nine biomes, which harbour ± 24 000 plant species accounting for 10 % of the world’s plants (Germishuizen & Meyer 2003, Rouget et al. 2004, SANBI South African National Biodiversity Institute 2007). Based on this rich plant diversity, Crous et al. (2006a) estimated that South Africa could harbour 171 500 species of fungi, many of which are probably endemic and most of which are currently undescribed. Considering the more recent published estimates of total fungal biodiversity, this estimate should be seen as conservative.
Fungi are being described faster today than at any time in prior history, but at the current rate it will take 1 000 years to describe all fungi estimated to exist, if not longer, considering the fungal lineages that are considered unculturable. Some argue for a change to the International Code of Nomenclature for Algae, Fungi and Plants ( Turland et al. 2018) to allow for sequence-based nomenclature, which would speed up the naming of fungi ( Lucking et al. 2021). However, morphology and more traditional taxonomic approaches remain important given many described fungi for which no reference sequences are available in public databases. Despite the immense opportunities available, South Africa and Africa as a continent lag behind others in the documentation and description of new fungal species, contributing only 9 % of the ± 2 000 species described annually ( Hawksworth & Lucking 2017, Antonelli et al. 2020).
The New and Interesting Fungi (NIF) series is published regularly in the journal Fungal Systematics and Evolution (FUSE; https://fuse-journal.org/ ). Its aim is to capture knowledge about the biodiversity of fungi and provide a platform for easier publication of new species, provide new records or DNA sequences of previously unsequenced species, and many other things mycologists find interesting. This issue is dedicated to Prof Michael J. (Mike) Wingfield on the occasion of his 70th birthday. As the founding director of the Forestry and Agricultural Biotechnology Institute (FABI) at the University of Pretoria (South Africa), he has dedicated his career as a forest pathologist to gain a better understanding of how fungi and insects affect forestry industries around the world and how these impacts can be mitigated. Mike has a deep love of nature, fungi and describing diversity. He has introduced 827 species names to date (Fig. 1 ), spanning across 233 genera. The remarkable aspect of this is that most of these species are associated with disease symptoms, and many are directly implicated in affecting global tree health.
Fig. 1.
A word cloud was generated using the 827 fungal names introduced by Prof Michael J. Wingfield. Data were obtained from MycoBank on 1 April 2024 and do not necessarily reflect current taxonomic classifications.
MATERIALS AND METHODS
Strains and specimens
Strains studied here are deposited in recognised culture collections around the world, including CBS (Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands), and CMW & CMW-IA (Forestry and Agricultural Biotechnology Institute, Pretoria, South Africa). Similarly, specimens were deposited at recognised fungaria, including CBS-H (Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands), PRU (H.G.W.J. Schweickerdt Herbarium, University of Pretoria, Pretoria, South Africa), VIC (Universidade Federal de Viçosa, Brazil), and the HFJAU (Fungarium of Fungi, Jiangxi Agricultural University, China).
DNA extraction, amplification (PCR) and phylogeny
Genomic DNA was extracted from fungal strains using standard procedures at the authors’ institutes. Fifteen gene regions were amplified with PCR and then Sanger sequenced in both directions, including the nrDNA internal transcribed spacer region ITS1-5.8S-ITS2 (ITS), the nrDNA large and small subunits (LSU and SSU), the partial ATP citrate lyase gene (acl), the partial actin gene (actA), the partial β-tubulin gene (BenA = tub2), the partial calmodulin gene (CaM = cmdA), the mitochondrial gene cytochrome oxidase subunit 3 (CO3), the gene coding for the theta subunit of the TCP-1 chaperonin complex gene (Cct8), the partial glyceraldehyde-3-phosphate dehydrogenase gene (gapdh), the partial histone H3 gene (his3), the partial DNA-directed RNA polymerase II largest subunit gene (RPB1), the partial DNA-directed RNA polymerase II second largest subunit gene (RPB2), two regions of the translation elongation factor 1-alpha gene (tef1), and a putative ribosome biogenesis protein gene (Tsr1). Sequences were deposited in NCBI’s GenBank nucleotide database (https://www.ncbi.nlm.nih.gov/genbank/) and accession numbers are listed in Table 1.
Each newly generated sequence was subjected to a BLAST search against the NCBI database ( https://blast.ncbi.nlm.nih.gov/Blast.cgi ) to identify closely related sequences that guided subsequent phylogenetic analyses. The phylogenetic approaches used are provided in appropriate figure legends below.
Morphology
Strains were inoculated onto various growth media, including Czapek yeast autolysate agar (CYA), CYA supplemented with 5 % NaCl (CYAS), creatine sucrose agar (CREA), dichloran-glycerol agar (DG18; Oxoid CM0729), oatmeal agar (OA), malt extract agar (MEA: Oxoid CM0059), malt extract yeast extract 10 % glucose 12 % NaCl agar (MY1012), malt yeast 50 % glucose agar (MY50G), potato dextrose agar (PDA), synthetic nutrient-poor agar (SNA), autoclaved pine needles on 2 % tap water agar (PNA) ( Smith et al. 1996), water agar (WA), and yeast extract sucrose agar (YES). Recipes for these were published in Samson et al. (2019). Plates were incubated at 25 °C for 2–4 wk, unless specified otherwise in descriptions. Colony colours (surface and reverse) were determined using the colour charts of Rayner et al. (1970) or Kornerup & Wanscher (1967). Colonies were captured with a Sony alpha 7 III camera equipped with a Sony FE 90 mm f/2.8 Macro G OSS lens (Tokyo, Japan) and a Zeiss AXIO Zoom.V16 dissection microscope equipped with a Zeiss AxioCaM 512 colour camera driven by Zen Blue v. 3.2 software (Carl Zeiss CMP, Göttingen, Germany). For some images, extended depth of field analyses was performed using Helicon Focus v. 7.5.4 (HeliconSoft, Kharkiv, Ukraine). Alternatively, a Nikon SMZ25 dissection microscope, and with a Zeiss Axio Imager 2 light microscope using a Nikon DS-Ri2 camera with associated software was used. Microscope slides were prepared from sporulating colonies mounting material in lactic acid, Shear’s mounting fluid or water.
RESULTS AND DISCUSSION
Phylogeny
BLAST results and phylogenetic trees are discussed below in the species notes where applicable. Statistics associated with those phylogenies are provided in the figure legends, while datasets used for analyses are summarised in Suppl. Tables S1–S12 and Fig. 1–S4. The supplementary material and alignments can be accessed at FigShare doi: 10.25403/UPresearchdata.26176783.
Taxonomy
Alternaria halotolerans Fotedar, Sand.-Den., Kolecka & Boekhout, sp. nov. MycoBank MB 854310.
[originally described as Alternaria halotolerans Fotedar et al., Persoonia 50: 213. 2023, nom. inval., Art. F.5.1 (Shenzhen)].
Etymology: Named after its ability to grow in hypersaline sea water with high salinity.
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Pleosporaceae.
DNA barcodes: ITS = KY387606, LSU = KY781812, gapdh = KY387604, tef1 (first part) = KY387608.
Description and illustration: Crous et al. (2023: 213).
Typus. Qatar, Doha, Inland Sea, Khor Al- Adaid, E51.3325 N24.55226, from hypersaline sea water at 2.5 m depth, 20 Sep. 2014, R. Fotedar [holotype CBS H-24902 (dried culture), culture ex-type 2M108 = QCC M0010/16 = CBS 146348].
Notes: Alternaria halotolerans was published with the identifier MB 844257, but this is the identifier of Nectriella adonidis ( Crous et al. 2022). The name is formally validated here.
Authors: M. Sandoval-Denis & T. Boekhout
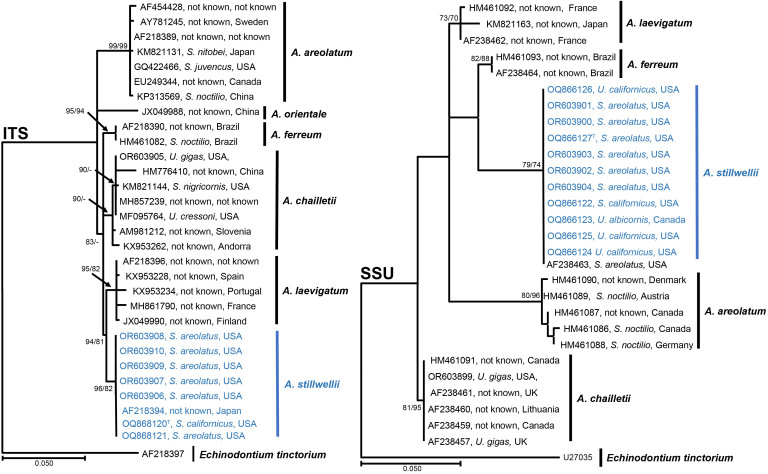
Amylostereum stillwellii Slippers, K.N.E. Fitza & J.D. Allison, sp. nov. MycoBank MB 854264. Figs 2, 3.
Fig. 2.
Phylogenetic tree based on Maximum Likelihood and Maximum Parsimony analysis of ITS and SSU. Maximum Likelihood analyses were performed using PhyML v.3.1 ( Guindon & Gascuel 2003, Darriba et al. 2012). PAUP v. 4.0b10 ( Maddison et al. 1997) was used for the Maximum Parsimony analyses. Bootstrap support values greater than 70 % are given at the nodes. Echinodontium tinctorium was used to root the tree. GenBank accession numbers are given in the tree and samples for the new species are in blue. Labels include the GenBank accession number, Siricid host and country of collection (− = support lower than 70 %).
Fig. 3.
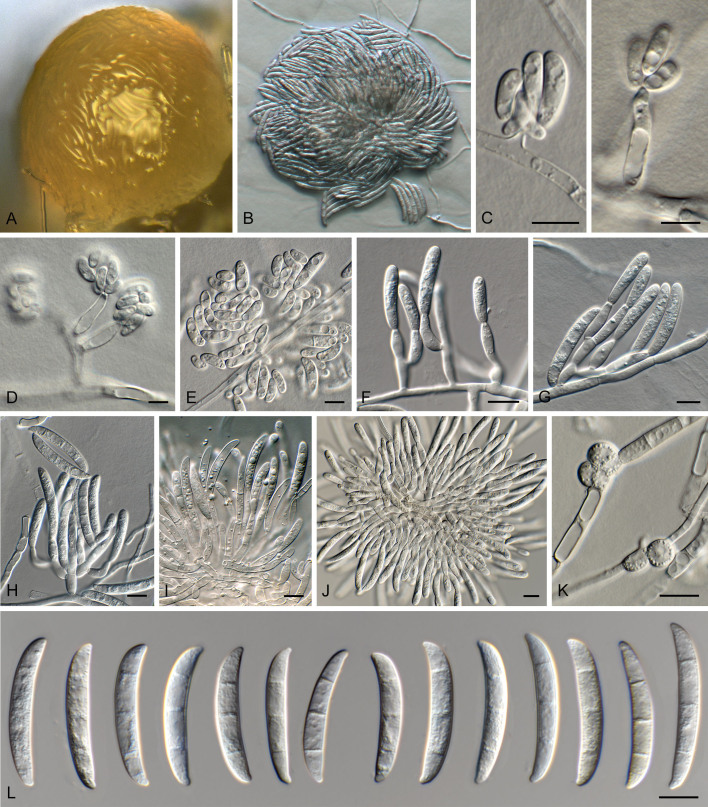
Amylostereum stillwellii. A. Typical site in California where S. areolatus, S. californicus and other Siricids carrying A. stillwellii occur (Photo credit Danny Cluck). B. Specimens of S. areolatus (top) and S. californicus (bottom). C. Colony on MEA after 4 wk. D. Hyphae with clamp connections. Scale bars = 10 μm.
Etymology: Named after Merlyn Arthur Stillwell, a Canadian entomologist who published a number of key papers on Siricidae in North America, who first isolated this species and who’s isolate is used to produce the type specimen for the description.
Classification: Basidiomycota, Agaricomycetes, Russulales, Amylostereaceae.
DNA barcodes: ITS = OQ868120, SSU = OQ866127.
Description: Colonies on MEA attaining 90-mm-diam after 14 d at both 20 and 25 °C. Growth observed at 10–25 °C, but no growth at 30 °C. Culture consists of white to creamy aerial mycelia; soluble pigments absent; exudates absent; reverse centre light orange (5A5) fading into light yellow (4A4). Hyphal system monomitic, consisting of richly branched, thin- to thick-walled generative hyphae, with clamps, 3–4 μm in width.
Habit, habitat & distribution: California and New Mexico in the USA and Ontario in Canada. Isolated from the mycangia of Sirex and Urocerus species.
Typus: USA, California, isolated from mycangia of Sirex areolatus, after 1970, M.A. Stillwell [holotype PRU(M) 4529, culture ex-holotype CMWIA 1508 = DAOMC 250356].
Additional materials examined: USA, California, from mycangia of S. areolatus in the Siricidae collection at the Great Lakes Forestry Centre (Canada), 1964, unknown collector, cultures DAOMC 250355 = CMW 61793, DAOMC 250357 = CMW61791, DAOMC 250358 = CMW 61790, DAOMC 250359 = CMW 61789.
Environmental DNA sequenced from mycangia of wasps in the Siricidae collection of the Great Lakes Forestry Centre (Canada): Canada, Ontario, from U. albicornis, 2015, unknown collector, AFC1I11. USA, California, from S. californicus, 2015, unknown collector, USA1A10; ibid., from U. areolatus, USA1A13 and unknown accession number; New Mexico, from Urocerus areolatus, 2015, unknown collector, USA1G1.
Notes: Amylostereum is a monotypic genus in the Amylostereaceae (Agaricomycetes), that is well known for its symbiotic relationship with woodwasps in the Siricidae (Hymenoptera) ( Slippers et al. 2003). Trees that are weakened due to fire or other abiotic or biotic factors are often attacked by Siricidae such as those from which the new Amylostereum species have been isolated ( Middlekauff 1960). A previous study identified an isolate from S. areolatus from California as potentially undescribed ( Slippers et al. 2000), and here we further characterise this and other related isolates from the region to describe it as Amylostereum stillwellii. This species is phylogenetically most closely related to A. laevigatum, but is clearly distinct from all species in this genus based on both ITS and SSU. Amylostereum stillwellii showed 97 % similarity in SSU sequence data to A. laevigatum (GenBank AF238462) and A. ferreum (GenBank AF238464) and 94 % to A. chailletti (GenBank KU870311) and A. areolatum (GenBank KM821153). The ITS locus of A. stillwellii showed 98 % similarity with A. laevigatum (GenBank JX049990), A. chailletti (GenBank MF095764), A. ferreum (GenBank HM461082) and 97 % similarity to A. areolatum (GenBank KM821131) and A. orientale (GenBank JX049988). Phylogenetic analysis of a subset of sequences of the ITS and SSU (Fig. 2 ), illustrate these relationships with bootstrap support ranging from 93 % to 99 %.
Authors: B. Slippers, K.N.E. Fitza & J.D. Allison
Angiopsora anthurii Pereira, Furtado & Barreto, sp. nov. MycoBank MB 854274. Figs 4, 5.
Fig. 4.
Consensus phylogram (50 % majority rule) obtained from a Bayesian analysis of the Crossopsoraceae and Phakopsoraceae alignment (Suppl. Table S1), combining ITS, LSU, and CO3 sequences. Bootstrap support values (BS) higher than 70 % and Bayesian posterior probabilities (PP) higher than 0.95 are shown at the nodes. The scale bar represents the expected changes per site. The order and families are indicated with coloured blocks to the right of the tree. The tree was rooted with Eocronartium muscicola (vouchers CFSZ 12722 and DB 1296) and the novelties treated here are indicated in bold face.
Fig. 5.
Angiopsora anthurii (VIC 49488). A. Symptoms caused by Angiopsora anthurii on a leaf of Anthurium andraeanum. B. Uredinia formed on the adaxial side of the leaf (arrow indicates an immature uredinium). C. Uredinia formed on the abaxial side of the leaf. D. Close-up view of a uredinium, showing urediniospores. E. Leaf section of a uredinium. F. Urediniospores. G. Uredinia in scanning electron microscope (SEM). H. Urediniospores in SEM. Scale bars: E, F = 50 μm; G, H = 20 μm.
Synonym: Uredendo anthurii Barrera-Enriquez & Salazar, Bol. Cient. Mus. Hist. Nat. U. de Caldas 23: 99. 2019. Nom. inval., Art. F.5.1 (Shenzhen).
Etymology: Named after the host from which it was collected, Anthurium.
Classification: Basidiomycota, Pucciniomycotina, Pucciniomycetes, Pucciniales, Uredinineae, Crossopsoraceae.
Uredinia amphigenous, mostly hypophyllous, subepidermal, erumpent, spores released through small cracks on host epidermis to form small yellow to orange aggregates, sparse or isolate, 0.2–0.6 mm diam, associated with orange to yellow
chlorotic areas on the upper side of the leaves. Urediniospores sessile, 21–38 × 18.5–26.5 μm, globose to ellipsoidal, to obovoid, pale brown to golden-yellow, rarely hyaline, spore wall 0.5–2 μm thick, echinulate, germ pores absent. Telia unknown.
Typus: Brazil, state of Minas Gerais, Viçosa, UEPE Belvedere-Floricultura (Universidade Federal de Viçosa campus), on leaves of Anthurium andraeanum (Araceae), 7 Jul. 2023, R.W. Barreto, RWB 2433 (holotype VIC 49488).
Additional material examined: Brazil, state of Minas Gerais, Viçosa, UEPE Belvedere-Floricultura (Universidade Federal de Viçosa campus), on leaves of Anthurium andraeanum, 25 Aug. 2023, C.M. Pereira, DOA 2356 (VIC 49490); ibid., Sítio Canteros, on leaves of Anthurium andraeanum, 15 Jul. 2023, R.W. Barreto, RWB 2434 (VIC 49489).
Notes: Through phylogenetic inferences, combining three genetic regions (ITS, LSU and CO3), specimens obtained from Anthurium andraeanum clustered within the genus Angiopsora. This genus was established by Mains (1934) for fungi that infect grasses (Poaceae) and resemble Phakopsora. Although Angiopsora was originally treated as a synonym for Phakopsora ( Cummins & Hiratsuka 2003), Aime & McTaggart (2021) found that Angiopsora is the proper name for the phakopsora-like fungi occurring on grasses.
Despite teliospores being considered a morphological marker for Angiopsora, telia were not observed in the material on A. andraeanum. Two rust species have been described on Anthurium spp., namely: Uredo anthurii (Saccardo 1895) and Uredendo anthurii (Barrera-Henriquez & Salazar-Yepes 2019; invalid, as a recognized repository was not cited in the protologue). Only the uredinial stage is known for either taxon.
Morphological features of the specimens under study are very similar to those described for Uredendo anthurii, and also close to those given by Hennen et al. (2005) and other authors for Uredo anthurii – including the fact that urediniospores are “borne without obvious pedicels”. Nevertheless, urediniospores are larger in Uredo anthurii [(30–)35–47(–52) × (28–)30–37 μm] than those described by Barrera-Henriquez & Salazar-Yepes (2019) as for our specimens. The absence of germ pores in the newly collected specimens from Brazil is an additional distinction. Hennen et al. (2005) described germ pores of Uredo anthurii as obscure or up to 4, more or less equatorial, but in our examinations, including under SEM, no germ pores were found. Here we interpret those differences as sufficient to place our fungus in what was previously known as Uredendo anthurii.
The new finding of A. anthurii in Brazil reported here on the commercially important A. andraeanum is significant. Furthermore, there are no previous reports of any member of Angiopsora causing rust on a plant belonging to the Araceae. This expands the known host range, at the family level, for this genus. The true generic placement of Uredo anthurii remains to be resolved.
Based on a megablast search of NCBI’s GenBank nucleotide database, the highest similarity found for ITS sequences were with Pucciniastrum agrimoniae [strain HMJAU8580, GenBank MG787121; Identities = 256/282 (91 %), three gaps (1 %)], Pucciniastrum agrimoniae [HMJAU8579, GenBank MG787120; Identities = 256/282 (91 %), three gaps (1 %)], and Austropuccinia psidii [UFV-12, GenBank EF210144; Identities = 256/283 (90 %), five gaps (1 %)]. Conversely, the closest hits for the LSU sequences for the rust on A. andraeanum were with Angiopsora chusqueae [R161, GenBank EU851156; Identities = 970/1 009 (96 %), four gaps (0 %)], Uredo musae [880738, Genbank KY764185; Identities = 971/1 026 (95 %), 16 gaps (1 %)], and Kweilingia divina [MCA2887, GenBank DQ354554; Identities = 1 037/1 099 (94 %), nine gaps (0 %)]. Closest hits for CO3 sequence were with Angiopsora paspalicola [UMS03, GenBank OQ200383; Identities = 548/556 (99 %), no gaps], Angiopsora paspalicola [UMS02, GenBank OQ200382; Identities = 548/556 (99 %), no gaps], and Angiopsora paspalicola [BRIP 55625, GenBank MW036496; Identities = 548/556 (99 %), no gaps].
Authors: C.M. Pereira, E.C.A. Furtado & R.W. Barreto
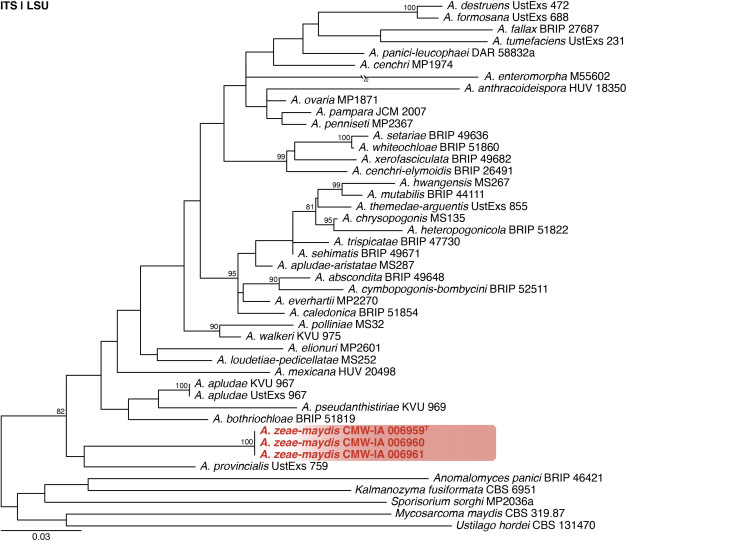
Anthracocystis zeae-maydis Visagie, sp. nov. MycoBank MB 854282. Figs 6, 7.
Fig. 6.
Multigene phylogeny of Anthracocystis based on ITS and LSU sequences (see Suppl. Table S2). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Each region was treated as separate partitions and the nucleotide substitution model GTR+I+G was applied to each ( Abadi et al. 2019). The tree was rooted to selected species from closely related genera in Ustilaginaceae. Anthracoystis zeae-maydis strains are shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Fig. 7.
Anthracocystis zeae-maydis. A. Colonies (left to right) on PDA and WA. B–D. Close-up of colonies on PDA (B), PDA at 30 °C (C) and WA (D). E. Yeast-like cells from slimy areas in colonies. F–K. Pseudohyphal cells. Scale bars: B–D = 5 mm; E–H = 10 μm.
Etymology: Latin, zeae-maydis, named after Zea mays (maize), the substrate this species was isolated from.
Classification: Basidiomycota, Ustilaginomycetes, Ustilaginales, Ustilaginaceae.
DNA barcodes: ITS = OR500016, LSU = PP375198.
Description (7 d): Colonies on PDA 25 °C 11–12 mm, creamy, wrinkly, yeast-like, slightly slimy to slimy centrally and filamentous at margin. Colonies on PDA 30 °C 9–10 mm, creamy, yeast-like, slightly slimy to slimy, some areas filamentous. No growth on PDA 37 °C. Colonies on WA 25 °C 15–16 mm, white to cream, flat, filamentous. Colonies on WA 30 °C 11–12 mm. No growth on WA 37 °C. Cells in yeast-like areas from polar budding on short stalks, blastoconidia cylindrical to fusiform, 3–9 × 1.5–3 μm. Pseudohyphae forming in filamentous areas, basal cells 11–30 × 1.5–3 μm, secondary cells 4–8 × 1.5–3 μm, blastoconidia fusiform, 2.5–3.5 × 1.5–3 μm.
Typus: South Africa, North West Province, Rostrataville (−26.79312, 25.69841), from pre-stored Zea mays (maize), 16 Jul. 2020, C.M. Visagie [holotype PRU(M) 4584, culture ex-type CMW-IA 006959 = CMW 64092 = CBS 152024 = CN054I3].
Additional materials examined: South Africa, North West Province, Rostrataville (−26.79312, 25.69841), from pre-stored Z. mays, 16 Jul. 2020, C.M. Visagie, culture CMW-IA 006960 = CMW 64093 = CBS 152025 = CN054I4; Gauteng Province, Raathsvlei, −26.5686, 27.6591, from pre-stored Z. mays, 16 Jul. 2020, C.M. Visagie, culture CMW-IA 006961 = CMW 64094 = CBS 152026 = CN055H1.
Notes: Anthracocystis was introduced by Brefeld (1912) with A. destruens [MB# 431380] as generic type. The smut fungi have been the focus of several taxonomic revisions using phylogenetic data ( McTaggart et al. 2012, Piątek et al. 2015, Wang et al. 2015). Morphological descriptions are usually based on the sexual morph directly observed from its plant hosts and typically does not include a description of its yeast form. Our strains were isolated from pre-stored maize kernels for which we did not observe a sexual morph and this complicates morphological comparisons. Strains of A. zeae-maydis form a unique, well supported branch in phylogenies based on ITS and LSU (Fig. 6 ). Pairwise comparisons revealed that the new species differs from the others by at least 75 bp for ITS and 10 bp for LSU.
Author: C.M. Visagie
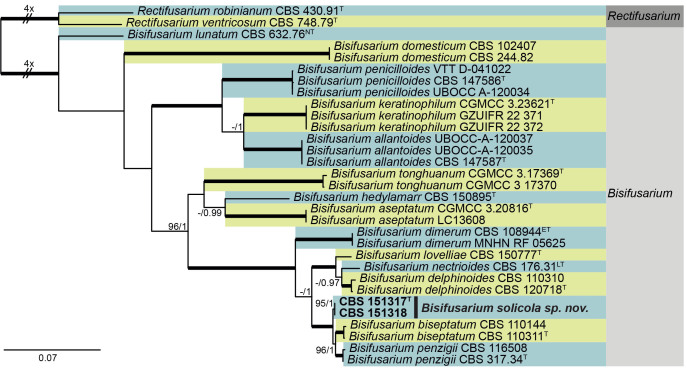
Bisifusarium solicola Crous & Sand.-Den., sp. nov. MB 854312. Figs 8, 9
Fig. 8.
Multigene phylogeny of Bisifusarium based on BenA, CaM, ITS, LSU, RPB2, and tef1 sequences (see Suppl. Table S3). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood (ML) tree was calculated in IQ-TREE v. 2.2.2.6 ( Minhet al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. Bayesian analysis was carried out in MrBayes v. 3.2.7a ( Ronquist et al. 2012), model selection according to MrModelTest v 2.3 ( Nylander 2004, Posada and Crandall 1998). The tree was rooted to Rectifusarium robinianum CBS 430.91 and R. ventricosum CBS 748.79. Values at nodes are ML ultrafast bootstrap (BS) ≥ 95% followed by Bayesian posterior probability (PP) ≥ 0.95. Bold branches indicate BS = 100 and PP = 1. Novel taxa are shown in bold. (ET = ex-epitype, NT = ex-neotype, LT = Lectotype, T = ex-type).
Fig. 9.
Bisifusarium solicola (CBS 151317). A–D. Sporodochia on SNA. E. Chlamydospores. F. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the fact that it was isolated from soil.
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Nectriaceae.
DNA barcodes: BenA = PP620571, CaM = PP620547, ITS = PP590160, LSU = PP590168, RPB2 = PP620557, tef1 = PP620565.
Description (7 d): Sporodochia formed on SNA and on carnation leaves, giving rise to a creamy conidial mass. Sporodochial conidiophores apically branched, consisting of primary and secondary cell giving rise to 1–3 conidiogenous cells, 20–30 × 3–4 μm. Sporodochial conidiogenous cells monophialidic, ampulliform to ellipsoid with tubular collarette, 1–3 μm tall, 2 μm diam, 10–15 × 3.5–4 μm. Sporodochial conidia falcate, gently dorsiventrally curved, with blunt apical cell and poorly developed foot-cell which is rarely present; 2-septate, with septa a third up from hilum and down from apex, not median, (15–)16–17(–18) × 4(–4.5) μm. Chlamydospores intercalary, solitary or in short chains, globose to ellipsoid, 6–10 μm diam.
Culture characteristics: Colonies flat, spreading with moderate aerial mycelium and smooth, lobate margins, reaching 60 mm diam after 7 d. On MEA surface centre brick, outer region rosy buff, and reverse centre umber, outer region sienna; on PDA surface centre saffron, outer region pale luteous, and reverse pale luteous; on OA surface buff.
Typus: South Africa, Western Cape Province, Cape Town, Kirstenbosch, soil adjacent to Agapanthus praecox, Nov. 2023, P.W. Crous (holotype CBS H-25369, culture ex-type CPC 47701 = CBS 151317).
Additional material examined: South Africa, Western Cape Province, Cape Town, Kirstenbosch, soil adjacent to Agapanthus praecox, Nov. 2023, P.W. Crous, culture CPC 47715 = CBS 151318.
Notes: Bisifusarium (formerly the F. dimerum species complex) is known to produce the PKS/NRPS hybrid siderophore, dimerumic acid (= dimerum acid) ( Diekmann 1970). The genus has characteristic (0–)1–2(–3)-septate macroconidia typically formed on sporodochia, and is commonly associated with human infections or isolated from desert soils, or foods with low water activity, like cheese (Schroers et al. 2009, Park et al. 2019, Crous et al. 2021a, Savary et al. 2023).
Bisifusarium solicola is closely related to B. biseptatum (2-septate conidia 15–22.5 × 2.5–4 μm) and B. penzigii [2-septate conidia (14–)18.5–21.5(–26) × (2.5–)3–4 μm; Schroers et al. 2009], but can be distinguished based on its conidial dimensions, and phylogeny (Fig. 6 ).
Authors: P.W. Crous & M. Sandoval-Denis
Cadophorella Crous & T. Hülsewig, gen. nov. MycoBank MB 854313.
Etymology: Name refers to its similarity to the genus Cadophora.
Classification: Leotiomycetes, Helotiales, Lachnaceae.
DNA barcodes: ITS = PP872394, LSU = PP872405, tef1 (second part) = PP874923.
Description: Mycelium consisting of hyaline, smooth, branched, septate hyphae. Conidiophores solitary, erect, subcylindrical, medium brown, smooth, branched above and below, 1–multiseptate. Conidiogenous cells terminal and intercalary, on short lateral branches, or solitary, medium to pale brown, smooth, subcylindrical to subulate, monophialidic, with prominent terminal collarette, constricted at base. Conidia solitary, aggregating in mucoid mass, aseptate, hyaline to olivaceous, smooth, obovoid with truncate hilum.
Type species: Cadophorella faginea Crous & T. Hülsewig
Cadophorella faginea Crous & T. Hülsewig, sp. nov. MycoBank MB 854314. Figs 10, 11.
Fig. 10.
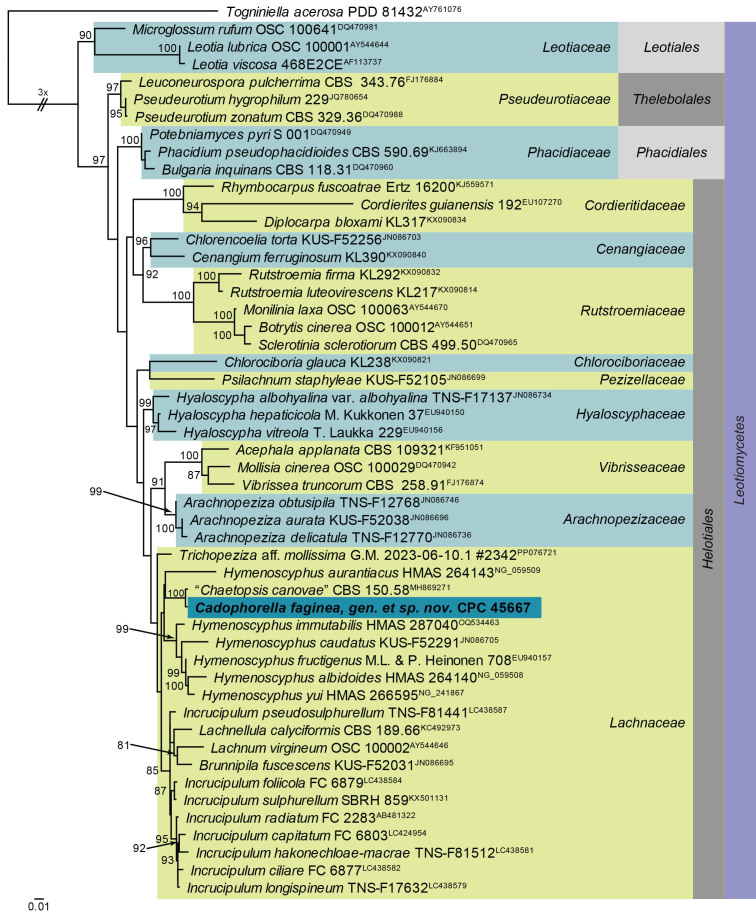
Maximum Likelihood (ML) phylogeny of Hymenoscyphus and allied genera based on the LSU nucleotide alignment. The dataset was aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and the ML tree was calculated in IQ-TREE v. 2.1.3 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. The tree was rooted to Togniniella acerosa (PDD 81432, GenBank AY761076). Values at nodes are ML ultrafast bootstrap ≥ 75 % (based on 10 000 replicates; values ≥ 95 % can be considered significant). The novel taxon is shown in bold and a dark blue block. Families, orders and the class are shown in coloured blocks on the right side of the phylogeny. The basal branch was shortened to facilitate layout.
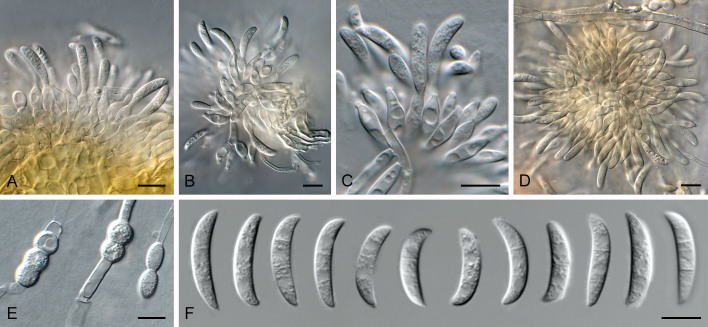
Fig. 11.
Cadophorella faginea (CPC 45667). A. Colony on SNA. B–G. Conidiophores and conidiogenous cells giving rise to conidia (note collarettes). H. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus from which it was isolated, Fagus.
Description (14 d): Mycelium consisting of hyaline, smooth, branched, septate, 2.5–3 μm diam hyphae. Conidiophores solitary, erect, subcylindrical, medium brown, smooth, branched above and below, 1–multiseptate, 15–120 × 2.5–3 μm. Conidiogenous cells terminal and intercalary, on short lateral branches, or solitary, 10–20 × 2.5–3 μm, medium to pale brown, smooth, subcylindrical to subulate, monophialidic, with prominent terminal collarette, constricted at base, 3–5 μm tall, apex 2.5–3 μm wide. Conidia solitary, aggregating in mucoid mass, aseptate, hyaline to olivaceous, smooth, obovoid with truncate hilum, 1 μm diam, 3–5 × 2–2.5 μm.
Culture characteristics: Colonies flat, spreading, surface folded with moderate aerial mycelium and smooth, lobate margin, reaching 35–45 mm diam after 2 wk at 25 °C. On MEA surface ochreous to umber and reverse umber; on PDA surface and reverse umber; on OA surface umber to ochreous.
Typus: Germany, North Rhine-Westphalia, Witten, recreation area Hohenstein, on dead capsule of Fagus sylvatica (Fagaceae), T. Hülsewig, 5 Oct. 2022, HPC 4129 = herbar.nr. 998 (holotype CBS H-25321, culture ex-type CPC 45667 = CBS 150804).
Notes: Cadophorella faginea represents a new cadophora-like genus occurring on capsules of Fagus sylvatica collected in Germany. Although not present on the material, there is a possibility that it represents the asexual morph of Hymenoscyphus fagineus, which is known from this substrate, but has no known asexual morph, and is presently not known from culture or DNA phylogeny, pending further collections. Cadophorella is not congeneric with Hymenoscyphus.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to “Chaetopsis canovae” [strain CBS 150.58, GenBank MH857732.1; Identities = 464/476 (97 %), no gaps], Hymenoscyphus immutabilis [strain SAT1324504, GenBank KY744162.1; Identities = 452/478 (95 %), three gaps (0 %)], Cudoniella acicularis [strain DSM 108380, GenBank MT154267.1; Identities = 424/453 (94 %), four gaps (0 %)], and Tricladium terrestre [voucher HKAS 128303, GenBank OR437505.1; Identities = 437/482 (91 %), nine gaps (1 %)]. Closest hits using the LSU sequence are “Chaetopsis canovae” [strain CBS 150.58, GenBank MH869271.1; Identities = 842/846 (99 %), no gaps], Trichopeziza aff. mollissima [voucher G.M. 2023-06-10.1 #2342, GenBank PP076721.1; Identities = 842/862 (98 %), no gaps], and Incrucipulum foliicola [voucher TNS:F-81508, GenBank LC438584.1; Identities = 840/863 (97 %), two gaps (0 %)]. Closest hits using the tef1 (second part) sequence had highest similarity to Leptodontidium irregulare [strain CBS 851.73, GenBank OQ454933.1; Identities = 403/432 (93 %), no gaps], Stipitochalara longipes [as Chalara longipes; strain CCF 3974, GenBank FR772047.1; Identities = 374/401 (93 %), two gaps (0 %)], and Flagellospora curvula [strain CCM F-18699, GenBank MK241453.1; Identities = 402/433 (93 %), two gaps (0 %)].
Authors: P.W. Crous, J.Z. Groenewald & T. Hülsewig
Devriesia mallochii Visagie & Seifert, sp. nov. MB 854283. Figs 12, 13.
Fig. 12.
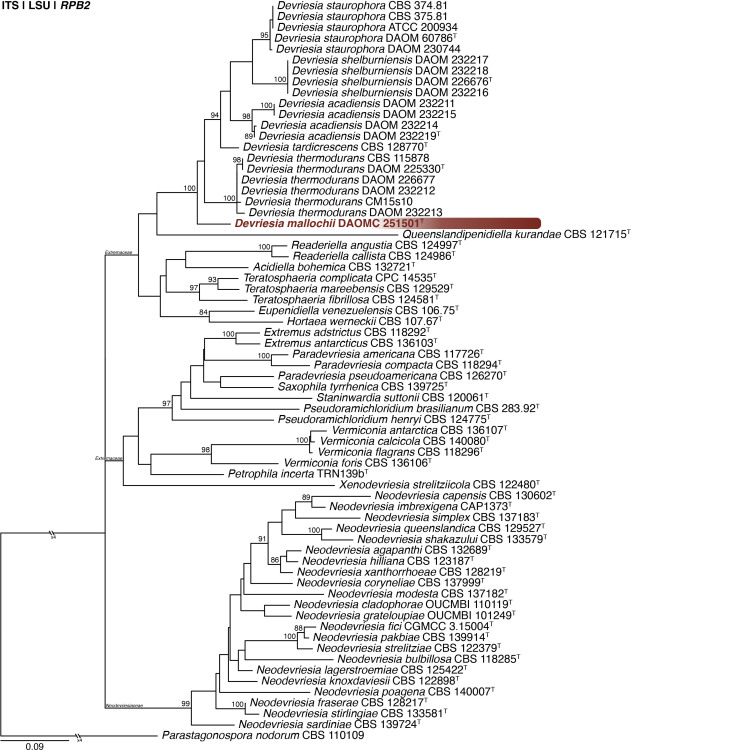
Multigene phylogeny of Devriesia and related genera based on ITS, LSU and RPB2 sequences (see Suppl. Table S4). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Each region was treated as separate partitions and the most appropriate nucleotide substitution model based on the Akaike information criterion was applied to each using PartitionFinder v. 2 ( Lanfear et al. 2017). The tree was rooted to Parastagonospora nodorum. Devriesia mallochii is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
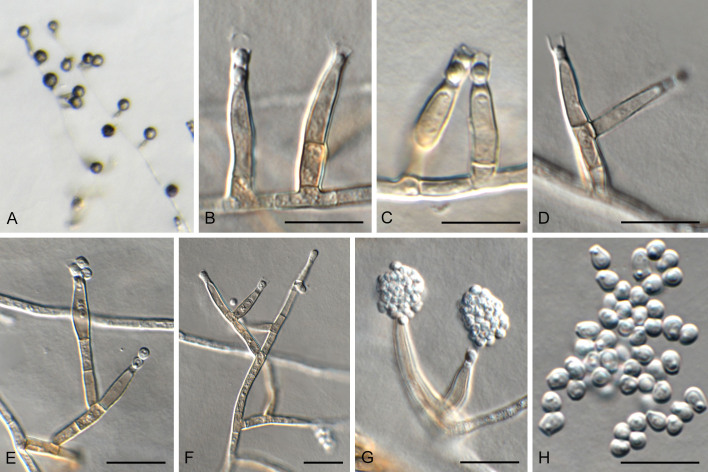
Fig. 13.
Devriesia mallochii. A, B. Colonies on OA. C. Hyphal growth on OA. D–I. Conidiophores. J, K. Conidia and chlamydospores. Scale bars: C = 50 μm; D–K = 10 μm.
Etymology: Latin, mallochii, named after the Canadian mycologist Dave Malloch, who lives in Little Lepreau, from where this species is described.
Classification: Dothideomycetes, Mycosphaerellales, Teratosphaeriaceae.
DNA barcodes: ITS = MF319661, LSU = MF319663, RPB2 = PP356396.
Colony diam (in mm, after 7 d, at 25 °C): MEA 4–5, PDA 4–5, OA 4–5, DG18 2–3; after 14 d on MEA 7–8, PDA 6–8, OA 6–7, DG18 4, MY10-12 no growth, MY50G no growth, MEA at 15 °C microcolonies, MEA at 20 °C 4, MEA at 30 °C microcolonies, MEA at 35 °C no growth.
Colony characters (25 °C, 14 d): Colonies on MEA moderately deep, crateriform, concentrically sulcate, margins entire, Dark Olive (1F3–3F3), Olive Grey (2F2) to Olive (2F6), reverse black.
Colonies on PDA moderately deep, crateriform, margins entire, very narrow, Dark Olive (1F3–3F3), Olive Grey (2F2) to Olive (2F6), reverse black. Colonies on OA low, plain, margins entire, subsurface, aerial mycelium sparse, Dark Olive (1F3–3F3), Olive Grey (2F2) to Olive (2F6), reverse black.
Micromorphology: Aerial hyphae branching, septate, becoming thallic, arthrospores pigmented, cylindrical, 2.5–7.5(–10) × 2–3 μm (5.0 ± 1.4 × 2.4 ± 0.3 μm). Spreading hyphae septate, embedded in media, pigmented, thick walled, swollen, 6–13(–16) × 2.5–6 μm (9 ± 2.5 × 4 ± 0.8 μm). Chlamydospores formed in agar after 7 d, present in aerial mycelia after 14 d, from terminal and intercalary cells, composed of 2–3 cells, outline more or less clavate, 1-septate, terminal cell globose to subglobose, dark pigmented, 6–9 μm (7.6 ± 0.7 μm), basal cell flat, usually lighter pigmented, 3.5–6 × 4.5–7.5 μm (4.5 ± 0.6 × 6.2 ± 0.8 μm).
Typus: Canada, New Brunswick, Little Lepreau, from house dust, Jan. 2015, coll. A. Walker, isol. C.M. Visagie (holotype DAOM 745791, culture ex-type DAOMC 251501 = CMW-IA 001081 = CMW 60405 = CN002H9 = KAS 6301).
Notes: Seifert et al. (2004) introduced Devriesia for Cladosporium staurophorum with D. staurophora as generic type. In the same study, they also described three heat-resistant species as D. acadiensis, D. shelburniensis and D. thermodurans and provided a new combination for Cladosporium chlamydosporum as D. chlamydospora. MycoBank lists 29 described Devriesia names. Quaedvlieg et al. (2014) noted that Devriesia is polyphyletic and therefore introduced Extremus (family Extremaceae) for D. antarctica and D. adstricta, and Neodevriesia (family Neodevriesiaceae) for D. hilliana and D. xanthorrhoeae. Later, Crous et al. (2019) introduced Paradevriesia (family Extremaceae) for D. americana, and Wang et al. (2017) provided combinations for several Devriesia species that phylogenetically belonged to other genera. This means that Devriesia currently contains only the four species originally introduced by Seifert et al. (2004) and D. tardicrescens, described by Crous & Groenewald (2011). Morphologically, Devriesia mallochii is most similar to D. chlamydospora, both producing 1-septate, dark pigmented chlamydospores that are not clover-shaped. However, our new species produces much smaller chlamydospores [6–9 vs (8−)10–43 μm] ( Seifert et al. 2004). In addition, D. mallochii lacks the cladosporium-like conidiophores and ramoconidia typical of other species, with only arthroconidia observed in culture. The phylogenetic analysis placed D. mallochii basal to the other Devriesia species, noting that this branch was poorly supported. The new species survived heat-shock treatment for 30 min at 75 °C ( Seifert et al. 2004). Pairwise comparisons revealed that the new species differs from the others by at least 34 bp for ITS, 9 bp for LSU and 52 bp for RPB2.
Authors: C.M. Visagie & K.A. Seifert
Ericboehmia thailandica (Jayasiri & K.D. Hyde) Gardiennet et al., Ascomycete.org 11: 175. 2019. Figs 14, 15.
Fig. 14.
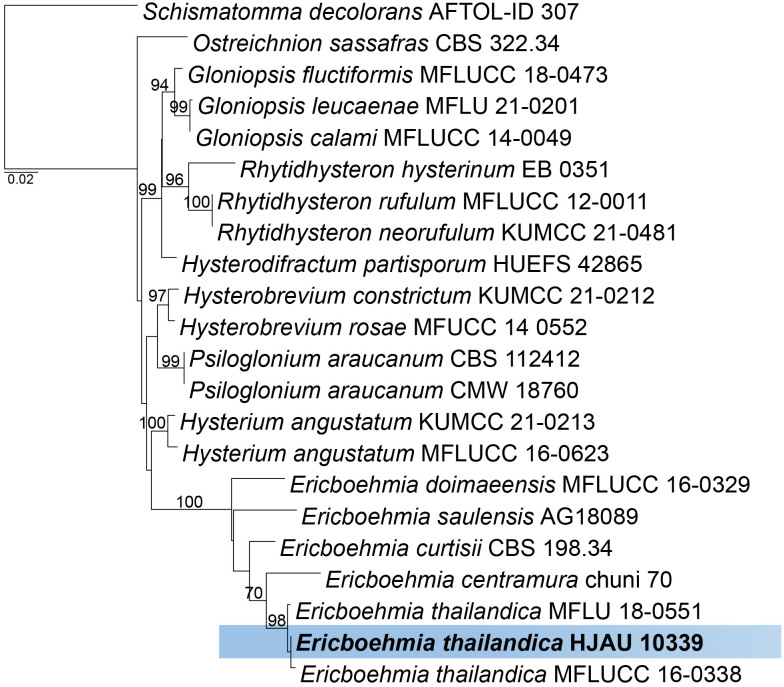
Phylogenetic analysis for Ericboehmia thailandica inferred from a maximum likelihood analysis of LSU/SSU sequences (see Suppl. Table S5). The analysis was performed with RAxML v. 8.2.12 ( Stamatakis 2014) using the rapid bootstrapping and search algorithm, with GTR+GAMMA nucleotide substitution model, and 1 000 bootstrap replicates. Maximum likelihood support values > 65 % are indicated on the branches. The tree is rooted with Schismatomma decolorans (AFTOL-ID 307). The species treated here is highlighted with bold face and indicated in a blue box. Scale bar on the tree indicates the expected number of changes per site. The matrix and the resulting tree have been deposited at TreeBASE (study ID: S31272).
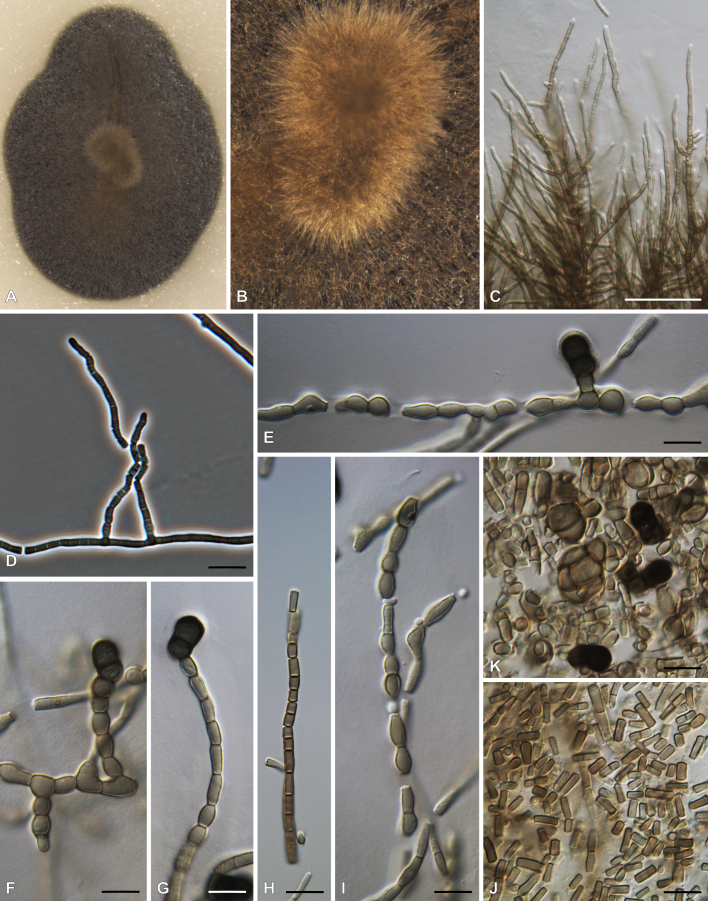
Fig. 15.
Ericboehmia thailandica (HFJAU 10339). A. Appearance of ascomata on host. B. Close-up of an ascoma. C. Section through ascoma. D. Pseudoparaphyses. E, F. Asci. G–L. Ascospores (ascospore stained in Indian ink showing a mucilaginous sheath in L). Scale bars: C = 100 μm; D–F = 80 μm; G–L = 25 μm.
Basionym: Hysterium thailandicum Jayasiri & K.D. Hyde [as ‘thailandica’], Mycosphere 9: 817. 2018.
Classification: Ascomycota, Dothideomycetes, Hysteriales, Hysteriaceae.
DNA barcodes: LSU = PP528529, SSU = PP543657.
Description: See Jayasiri et al. (2018).
Material examined: China, Yunnan Province, on dead leaf of Ficus benjamina (Moraceae), 17 Mar. 2016, D.S. Tennakoon, HFJAU 10339.
Notes: Ericboehmia thailandica was introduced by Jayasiri et al. (2018) from a dead stem of an unknown plant collected in Thailand. Subsequently, it was reported from a submerged mangrove wood (Rhizophoraceae) sample in Thailand ( Dayarathne et al. 2020). Initially, this was placed in Hysterium, but later transferred to Ericboehmia ( Gardiennet et al. 2019). Here we report it from a dead leaf of Ficus benjamina (Moraceae) collected in China.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the LSU sequence had highest similarity to Ericboehmia thailandica [strain MFLU 18-0551, GenBank MN017832.1; Identities = 880/882 (99.88 %), one gap (0.1 %)], E. thailandica [strain MFLUCC 16-0338, GenBank MH535895.1; Identities = 879/882 (99.76 %), two gaps (0.2 %)], E. centramura [strain chuni 70, GenBank KM272256.1; Identities = 877/882 (99.53 %), four gaps (0.4 %)], E. curtisii [strain CBS 198.34, GenBank FJ161176.2; Identities = 870/882 (98.64 %), two gaps (0.2 %)], E. saulensis [strain AG18089, GenBank MN338581.1; Identities = 850/882 (96.39 %), nine gaps (1 %)]. The closest hits using the SSU sequence are Hysterobrevium constrictum [strain KUMCC 21-0212, GenBank OK442652.1; Identities = 729/739 (98.7 %), four gaps (0.5 %)], H. mori [strain EB 0304, GenBank FJ161164.2; Identities = 729/739 (98.7 %), three gaps (0.4 %)], Psiloglonium sp. [strain GMB1090, GenBank OM836771.1; Identities = 720/739 (97.5 %), 35 gaps (4.7 %)], E. curtisii [strain CBS 198.34, GenBank FJ161137.2; Identities = 680/739 (92.05 %), 28 gaps (3.7 %)]. Based on the phylogenetic analysis (Fig. 14 ), our collection (HFJAU 10339) grouped with other Ericboehmia thailandica isolates (MFLUCC 16-0338 and MFLU 18-0551) as sister species to E. centramura.
Authors: D.S. Tennakoon & D.M. Hu
Fusarium kirstenboschense Crous & Sand.-Den., sp. nov. MycoBank MB 854315. Figs 16, 17.
Fig. 16.
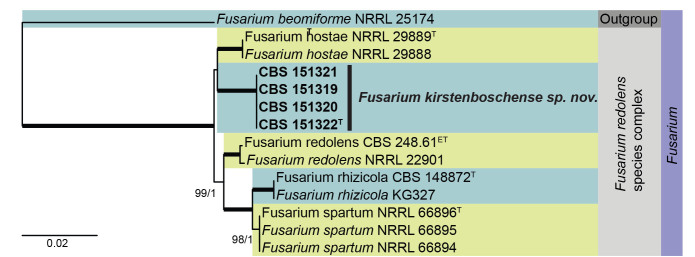
Multigene phylogeny of the Fusarium redolens species complex based on ITS, RPB1, RPB2, and tef1 sequences (see Suppl. Table S6). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood (ML) tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. Bayesian analysis was carried out in MrBayes v. 3.2.7a ( Ronquist et al. 2012), model selection according to MrModelTest v 2.3 ( Nylander 2004, Posada and Crandall 1998). The tree was rooted to Fusarium beomiforme NRRL 25174. Values at nodes are ML ultrafast bootstrap (BS) ≥ 95% followed by Bayesian posterior probability (PP) ≥ 0.95. Bold branches indicate BS = 100 and PP = 1. Novel taxa are shown in bold. (ET = ex-epitype, T = ex-type).
Fig. 17.
Fusarium kirstenboschense (CBS 151322). A, B. Sporodochia on SNA. C–E. Conidiogenous cells giving rise to microconidia. F–J. Conidiophores and conidiogenous cells giving rise to macroconidia. K. Chlamydospores. L. Macroconidia. Scale bars = 10 μm.
Etymology: Name refers to the location it was isolated from, Kirstenbosch, South Africa.
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Nectriaceae.
DNA barcodes: ITS = PP590165, RPB2 = PP620562, tef1 = PP620570, RPB1 = PP620554.
Description (7 d): Sporodochia erumpent, with creamy conidial mass on agar surface, and carnation leaves. Sporodochial conidiophores extensively branched, 30–80 × 5–6 μm, bearing terminal monophialides. Sporodochial conidiogenous cells monophialidic, subcylindrical, 12–20 × 3.5–4 μm, smooth, thin-walled, with apical collarette. Sporodochial conidia falcate, gently dorsiventrally curved, with blunt apical cell; basal cell with poorly developed foot-cell, 3-septate, (30–)32–36(–38) × 5(–6) μm. Chlamydospores sparse, solitary, globose, 8–10 μm diam. Microconidiophores reduced to monophialides, on aerial mycelium and submerged in agar, subcylindrical, 7–15 × 3–4 μm, giving rise to mucoid mass of microconidia. Microconidia reniform to fusoid-ellipsoid, 0–1-septate, guttulate, (7–)10–15(–20) × (4–)5–6 μm.
Culture characteristics: Colonies flat, spreading with moderate aerial mycelium and smooth, lobate margins, covering dish after 7 d. On MEA surface buff, reverse saffron; on PDA surface and reverse buff; on OA surface buff, with age turning orange.
Typus: South Africa, Western Cape Province, Cape Town, Kirstenbosch, soil adjacent to Agapanthus praecox, Nov. 2023, P.W. Crous (holotype CBS H-25370, culture ex-type CBS 151322).
Additional materials examined: South Africa, Western Cape Province, Cape Town, Kirstenbosch, soil adjacent to Agapanthus praecox, Nov. 2023, P.W. Crous, cultures CBS 151319–151321.
Notes: Fusarium kirstenboschense represents a new species in the Fusarium redolens species complex, being closely related to F. hostae (occurring on Hosta spp., Hostaceae, South Carolina, USA). Fusarium hostae is distinguished from F. kirstenboschense in having (2–)3(–4)-septate macroconidia with curved or hooked apical cells, poorly to well-developed foot cells, and fusoid 0(–2)-septate microconidia, and abundant chlamydospores ( Leslie & Summerell 2006).
Authors: P.W. Crous & M. Sandoval-Denis
Fusarium proliferatum (Matsush.) Nirenberg ex Gerlach & Nirenberg, Mitt. Biol. Bundesanst. Land-Forstw. 209: 309. 1982. Figs 18, 19.
Fig. 18.
Multigene phylogeny of the Fusarium fujikuroi species complex based on RPB2, and tef1. Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood (ML) tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. Bayesian analysis was carried out in MrBayes v. 3.2.7a ( Ronquist et al. 2012), model selection according to MrModelTest v 2.3 ( Nylander 2004, Posada and Crandall 1998). The tree was rooted to Fusarium nirenbergiae CBS 744.97. Grey coloured boxes indicate biogeographic groups according to O’Donnell et al. (1998). Values at nodes are ML ultrafast bootstrap (BS) ≥ 95% followed by Bayesian posterior probability (PP) ≥ 0.95. Bold branches indicate BS = 100 and PP = 1. Fusarium proliferatum is shown in bold. (ET = ex-epitype, NT = ex-neotype T = ex-type). The tree is based on accession numbers as indicated in Yilmaz et al. (2021).
Fig. 19.
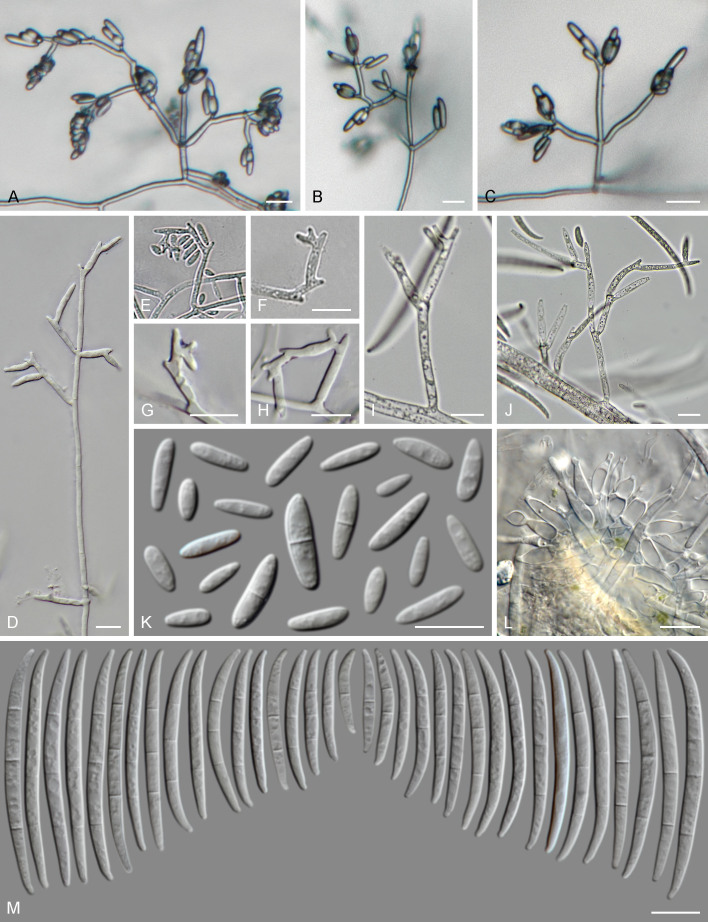
Fusarium proliferatum (A, D–H. CPC 39670. B, C. CPC 39666. K–M. CBS 480.96). A–J. Aerial conidiophores and conidiogenous cells. K. Microconidia. L. Sporodochial conidiophores. M. Sporodochial conidia (macroconidia). Scale bars = 10 μm.
Basionym: Cephalosporium proliferatum Matsush., Microfungi of the Solomon Islands and Papua-New Guinea: 11. 1971.
Synonyms: Fusarium proliferatum (Matsush.) Nirenberg, Mitt. Biol. Bundesanst. Land-Forstw. 169: 38. 1976. Nom. inval., Art. 41.3.
Fusarium proliferatum var. minus Nirenberg, Mitt. Biol. Bundesanst. Land-Forstw. 169: 43. 1976. Nom. inval., Art. 41.3.
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Nectriaceae.
DNA barcodes: BenA = MN534129, CaM = MN534217, RPB2 = MN534272, tef1 = MN534059.
Cultures examined: Papua New Guinea, Morobe province, Bulolo, forest soil, Nov. 1995, coll. A. Aptroot, isol. A. van Iperen [epitype CBS 480.96 (metabolically inactive) designated in Yilmaz et al. (2021), culture exepitype CBS 480.96 = IAM 14682 = NRRL 26427 = NY007.B6]. Vietnam, Lai Châu Province, Tam Đuong District, Musa itinerans (seed stored in Millenium Seed Bank), 2015, coll. unknown, isol. R. Hill, culture 880264-12 = Fus 4 = CPC 39666; Nghe An Province, Thanh Chuong District, Musa balbisiana (seed stored in Millenium Seed Bank), 2014, coll. unknown, isol. R. Hill, culture 836489-13 = Fus 7 = CPC 39670.
Notes: The epitypification of Cephalosporium proliferatum (basionym of Fusarium proliferatum) by Yilmaz et al. (2021) has resulted in a lot of discussion, as at the time, only a single strain was available for study. The proposed epitype (CBS 480.96, a metabolically inactive culture), has the same geographical origin and substrate as the holotype (Papua New Guinea, from forest soil) and was described as showing the typical features attributed to the prevailing concept of F. proliferatum. Unfortunately, these morphological features were not made evident in the accompanying illustrations (fig. 9, in Yilmaz et al. 2021).
A morphological re-examination under different culture conditions was subsequently conducted using the ex-epitype culture and two additional strains (CPC 39666 and CPC 39670, obtained as seed endophytes from Musa spp.). The new strains were identified as F. proliferatum based on a phylogenetic analysis of combined RPB2 and tef1 sequences (Fig. 16 ). The three studied strains showed distinctive monoand polyphialides proliferating sympodially, from which ovate to clavate, 0(–1)-septate microconidia with flat and rounded bases were produced, and accumulated into small false-heads on the conidiogenous cell apices (Fig. 17 ). These features are concordant with Matsushima’s original description of C. proliferatum (Matsushima 1971); but differ from the modern concept of F. proliferatum by lacking conidial chains and pyriform conidia. Based on the assumption that flat-based microconidia were an indication of the presence of conidial chains, Nirenberg (1976) and Gerlach & Nirenberg (1982) described this feature when recombining C. proliferatum in Fusarium, with Fusarium moniliforme (pro parte) as a synonym and considering F. moniliforme var. majus, F. moniliforme var. erumpens and F. moniliforme var. fici as probable synonyms. While all the taxa listed above are characterised by conidial chains, and in some cases also by forming pyriform conidia, this differs from Matsushima’s original description (“...Phialospores obovate, base more or less truncate, aseptate, 5–11 × 2–4 μm, hyaline, collected in a thin hyaline mucous mass at the collar of the polyphialide.”) With microconidia measuring 4.5–12 × 2–4 μm (ex-epitype), the presence of geniculate, proliferating polyphialides and the same substrate and location, the proposed epitype of C. proliferatum fits the original description of C. proliferatum, also by lacking conidial chains and pyriform conidia.
The presence of typical morphological features was noted to differ between strains according to the culture conditions, as striking polyphialides were common in cultures on CLA and OA plates incubated in darkness in CPC 39666 and CPC 39670, but less so when incubated under near-UV light, and were noticeably less common in CBS 480.96.
Authors: M. Sandoval-Denis, N. Yilmaz, E. Gaya, R. Hill & P.W. Crous
Hobsonia mirabilis (Peck) Linder, Ann. Missouri Bot. Gard. 16: 340. 1929. Figs 20, 21.
Fig. 20.
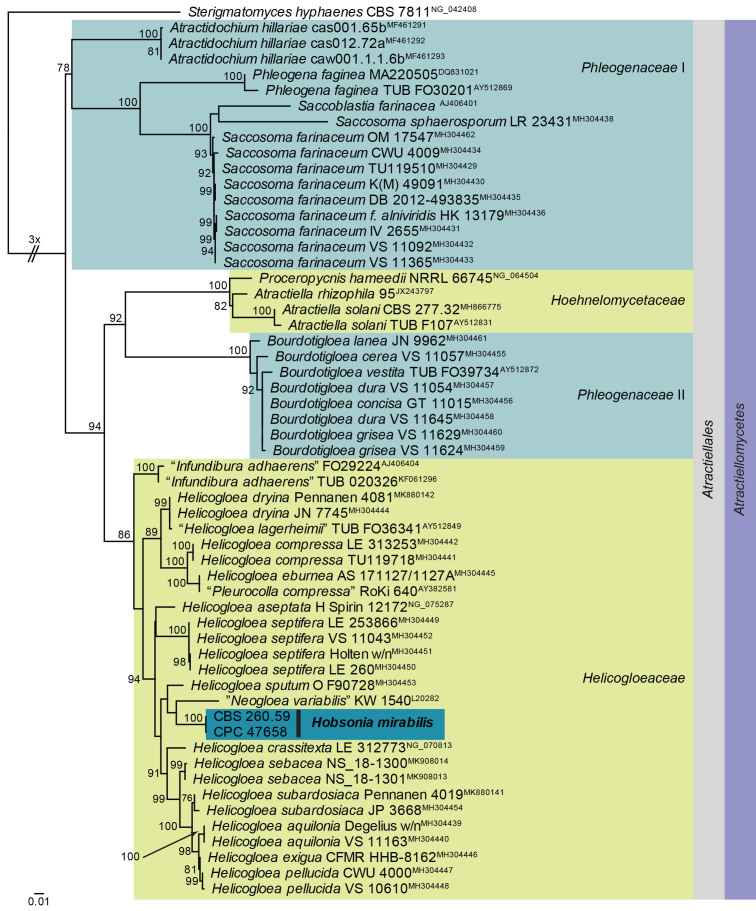
Maximum Likelihood (ML) phylogeny of Helicogloeaceae and allied families based on the LSU nucleotide alignment. The dataset was aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and the ML tree was calculated in IQ-TREE v. 2.1.3 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. The tree was rooted to Sterigmatomyces hyphaenes (CBS 7811, GenBank NG_042408). Values at nodes are ML ultrafast bootstrap ≥ 75 % (based on 10 000 replicates; values ≥ 95 % can be considered significant). The novel taxon is shown in bold and a dark blue block. Families, the order and the class are shown in coloured blocks on the right side of the phylogeny. The basal branch was shortened to facilitate layout.
Fig. 21.
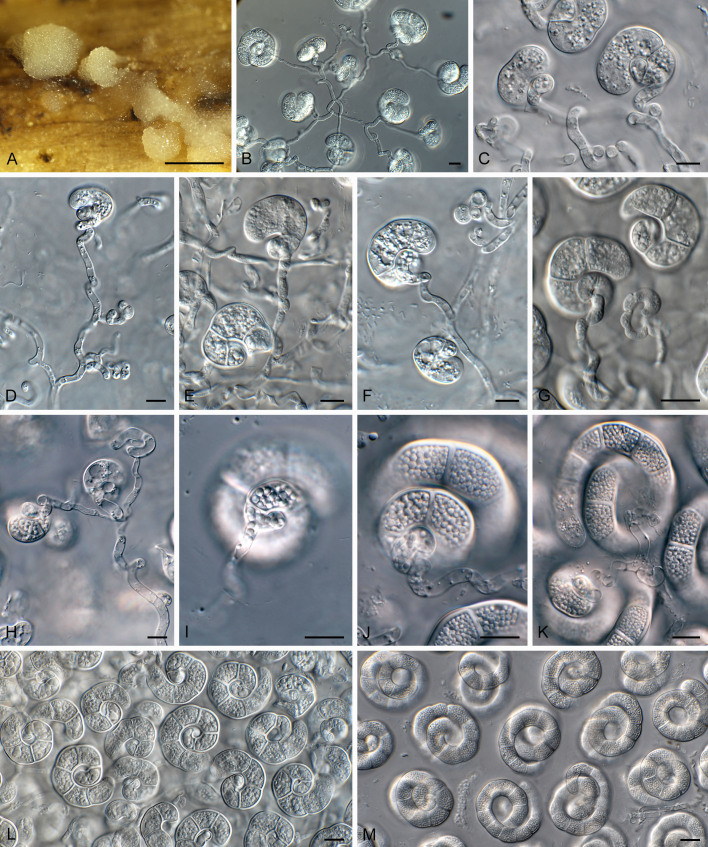
Hobsonia mirabilis (CPC 47658). A. Sporodochia on flower stalk. B–H. Conidiophores and conidiogenous cells giving rise to conidia. I–K. Conidiogenous cells with central attachment to conidia. L, M. Coiled conidia. Scale bars: A = 5 mm; B–M = 10 μm.
Basionym: Helicomyces mirabilis Peck, Annual Rep. New York State Mus. Nat. Hist. 34: 46. 1883 (1881).
Classification: Basidiomycota, Pucciniomycotina, Atractiellomycetes, Atractiellales, Helicogloeaceae.
DNA barcodes: ITS = PP872396, LSU = PP872407.
Description (14 d): Sporodochia superficial, erumpent, mucoid, globose, 1–7 mm diam, cream-coloured. Conidiophores arising from basal stroma, hyaline, smooth, subcylindrical, multiseptate, branched, 3–4 μm diam with conidiogenous cells terminal and intercalary. Conidiogenous cells subcylindrical, curved to coiled, hyaline, smooth, 10–20 × 3–4 μm, giving rise to solitary conidia (throughout sporodochium, at all levels), to which they are attached centrally, with conidium developing in coils that curl around the central attachment point (conidiogenous cells). Conidia hyaline, smooth, extensively guttulate (lipid droplets), thick-walled (1.5–2 μm diam), not encased in mucoid sheath (mounted in water, lactic acid, shears or Melzer), coiled 3–4 times, filaments (10–)13–15 μm diam, apical cell obtusely rounded, conidia 50–65 μm wide, 25–40 μm tall, 6–13-septate; smallest coil basal, widest coil apical or subapical (mounted in water). Sterile filaments not observed.
Material examined: South Africa, Western Cape Province, Cape Town, Kirstenbosch, on dead flower stalks of Agapanthus praecox (Amaryllidaceae), Nov. 2023, P.W. Crous & K.L Crous, HPC 4323 (CBS H-25368, cultures CPC 47658 = CBS 151610, CPC 47659).
Notes: Hobsonia resembles the genus Everhartia (type species E. hymenuloides), which has conidia that are 13–18(–20) μm diam, 2–2.5 times coiled, filaments 2.5–3.5 μm diam. In Everhartia conidia are attached via the basal cell to the conidiogenous cell, with sterile filaments similar in length to the conidiophores that give rise to terminal conidia (Moore 1953). Hobsonia is distinguished by its conidiogenesis (attached centrally to conidia, not basally), with conidiogenous cells intercalary and terminal, and lacking sterile filaments.
Hobsonia (based on H. gigaspora) differs from H. mirabilis in that the latter has non-gelatinous colonies, conidiophores are less frequently branched in H. mirabilis, but subdichotomously branched in H. gigaspora ( Linder 1929). Phylogenetically, it clusters among species of Helicogloea, which appears to be paraphyletic.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Helicogloea aseptata [voucher H Spirin 12172, GenBank NR_171842.1; Identities = 516/633 (82 %), 50 gaps (7 %)], Helicogloea eburnea [voucher AS 171127/1127A, GenBank MH304487.1; Identities = 400/498 (80 %), 43 gaps (8 %)], and Leucogloea compressa [voucher NAMA 2015-068 Mushroom Observer # 247459, GenBank MH910596.1; Identities = 395/496 (80 %), 38 gaps (7 %)]. The ITS sequence differs with a single nucleotide from that of Hobsonia mirabilis culture CBS 260.59 (563/564 nucleotides). Closest hits using the LSU sequence are Helicogloea sebacea (voucher NS_18-1301, GenBank MK908013.1; Identities = 777/820 (95 %), four gaps (0 %)), Helicogloea septifera (voucher LE 253866, GenBank MH304449.1; Identities = 774/818 (95 %), one gap (0 %)), and Helicogloea sputum (voucher O F90728, GenBank MH304453.1; Identities = 743/787 (94 %), three gaps (0 %)). The LSU sequence is identical to that of Hobsonia mirabilis culture CBS 260.59 (754/754 nucleotides).
Authors: P.W. Crous & J.Z. Groenewald
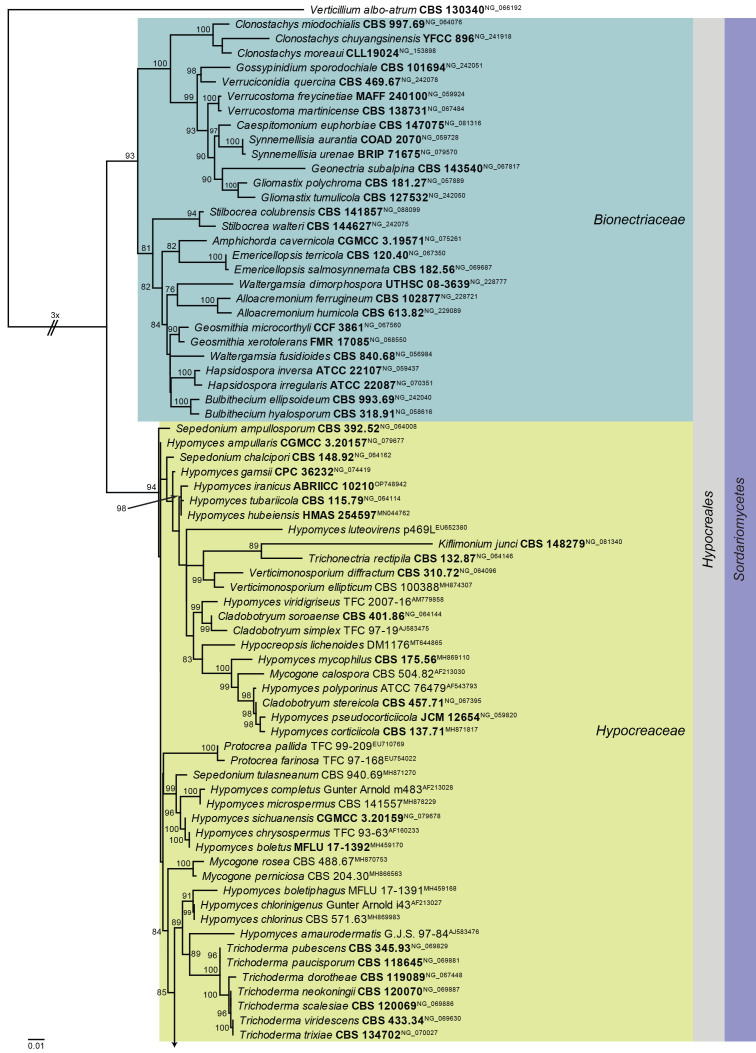
Hypomyces sympodiophorus Rogerson & Samuels. Mycologia 85: 268. 1993. Figs 22, 23.
Fig. 22.
Maximum Likelihood (ML) phylogeny of Hypomyces and allied genera based on the LSU nucleotide alignment. The dataset was aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and the ML tree was calculated in IQ-TREE v. 2.1.3 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. The tree was rooted to Verticillium albo-atrum (CBS 130340, GenBank NG_066192). Values at nodes are ML ultrafast bootstrap ≥ 75 % (based on 10 000 replicates; values ≥ 95 % can be considered significant). The novel taxon is shown in bold and a dark blue block, and sequences from material with a type status are indicated with a bold culture or voucher number. Families, the order and the class are shown in coloured blocks on the right side of the phylogeny. The basal branch was shortened to facilitate layout.
Fig. 23.
Hypomyces sympodiophorus (CPC 45631). A, B. Colony on SNA. C–F. Conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Synonyms: Sympodiophora stereicola G.R.W. Arnold, Nova Hedwigia 19: 302. 1970.
Cladobotryum stereicola (G.R.W. Arnold) Rogerson & Samuels, Mycologia 85: 268. 1993.
Helminthophora uniseptata R.F. Castañeda, Fungi Cubense (La Habana): 9. 1986.
Cladobotryum uniseptatum (R.F. Castañeda) K. Põldmaa, Mycologia 91: 192. 1999.
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Hypocreaceae.
DNA barcodes: ITS = PP872397, LSU = PP872408, RPB2 = PP874917, tef1 (second part) = PP874924.
Description (14 d): Mycelium consisting of hyaline, smooth, branched, septate, 2 μm diam hyphae. Conidiophores arising from superficial hyphae, verticillate, branched or not, with one to several whorls of phialides, 60–250 μm tall, 2–3 μm diam. Conidiogenous cells arranged solitary or in whorls, subcylindrical with slight apical taper, hyaline, smooth, 20–40 × 2–3 μm, apex 1.5 μm diam, collarette minute, not flared. Conidia solitary, hyaline, smooth, guttulate, slightly clavate, medianly 1-septate, tapering in basal cell to truncate hilum, 0.5 μm diam, (13–)14–16(–18) × (4–)5–6 μm.
Culture characteristics: Colonies erumpent, spreading, with abundant aerial mycelium and feathery, lobate margin, reaching 60 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface dirty white, and reverse pale luteous.
Typus: Germany, North Rhine-Westphalia, Witten, on Stereum sp. on unknown stem, 24 Jan. 2023, T. Hülsewig, HPC 4145 = herbar.nr. 1081 (CBS H-25316, culture CPC 45631 = CBS 150799).
Notes: Hypomyces parasitises sporocarps of different macromycetes (mostly on Basidiomycota, less often on Ascomycota representatives) ( Zare & Gams 2016). Hypomyces has superficial or immersed, ovoid to pyriform, yellow to red or green perithecia in a subiculum, 8-spored, subcylindrical asci, and ellipsoid to fusoid, 0–1(–3)-septate, hyaline, smooth to verrucose ascospores, and Cladobotryum asexual morphs ( Rossman et al. 1999). Based on the LSU phylogeny, the present collection is similar to the cosmopolitan species Hypomyces sympodiophorus (Australia, Europe, Thailand and the USA on Stereum spp.), but can be distinguished in that the latter has smaller conidia (oblong to ellipsoid, 1-septate, 10–15 × 5–6 μm; Rogerson & Samuels 1993). Based on preliminary analyses, H. sympodiophorus appears to be a species complex, but a more detailed study is required to resolve the various species occurring on Stereum spp.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Hypomyces xyloboli [strain CBS 110280, GenBank NR_160212.1; Identities = 418/446 (94 %), ten gaps (2 %)], Hypomyces albidus [strain CBS 460.71, GenBank MH860220.1; Identities = 399/437 (91 %), 18 gaps (4 %)], and Cladobotryum pinarense [strain CBS 400.86, GenBank MH861973.1; Identities = 404/443 (91 %), 20 gaps (4 %)]. Closest hits using the LSU sequence are Hypomyces sympodiophorus [strain CBS 403.86, GenBank AF160245.1; Identities = 850/850 (100 %), no gaps], Cladobotryum apiculatum [strain CBS 174.56, GenBank MH869109.1; Identities = 859/870 (99 %), no gaps], and Hypomyces armeniacus [strain TFC 95-154, GenBank AF160239.1; Identities = 839/850 (99 %), no gaps]. Closest hits using the RPB2 sequence had highest similarity to Cladobotryum protrusum [strain CBS 118999, GenBank FN868662.1; Identities = 659/771 (85 %), two gaps (0 %)], Hypomyces samuelsii [strain G.A. i1716, GenBank FN868701.1; Identities = 655/770 (85 %), no gaps], and Cladobotryum asterophorum [strain 2020010406-2, GenBank MW193555.1; Identities = 655/774 (85 %), no gaps]. Closest hits using the tef1 (second part) sequence had highest similarity to Trichoderma gelatinosum [strain S456, GenBank KJ665499.1; Identities = 429/457 (94 %), no gaps], Trichoderma tomentosum [strain S435, GenBank KJ665763.1; Identities = 428/457 (94 %), no gaps], and Trichoderma thelephoricola [strain S572, GenBank KJ665752.1; Identities = 428/457 (94 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & T. Hülsewig
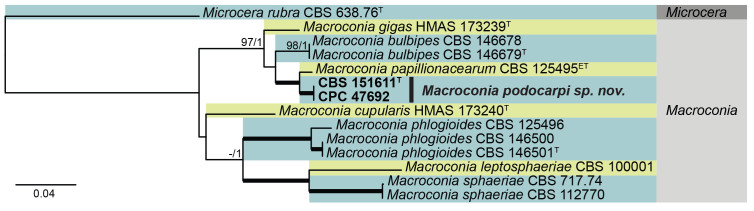
Macroconia papilionacearum (Seaver) Gräfenhan & Seifert. Stud. Mycol. 68: 102. 2011. Figs 24, 25.
Fig. 24.
Multigene phylogeny of Macroconia based on Acl, BenA, CaM, ITS, LSU, RPB1, and RPB2 sequences (see Suppl. Table S7). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood (ML) tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. Bayesian analysis was carried out in MrBayes v. 3.2.7a ( Ronquist et al. 2012), model selection according to MrModelTest v 2.3 ( Nylander 2004, Posada and Crandall 1998). The tree was rooted to Microcera rubra CBS 638.76. Values at nodes are ML ultrafast bootstrap (BS) ≥ 95% followed by Bayesian posterior probability (PP) ≥ 0.95. Bold branches indicate BS = 100 and PP = 1. Novel taxa are shown in bold. (ET = ex-epitype, T = ex-type).
Fig. 25.
Macroconia papilionacearum (CBS 125495). A. Red perithecia on twig in vivo. B, C. Conidia. D, E. Ascospores. Scale bars: A = 250 μm; B–E = 10 μm.
Basionym: Nectria papilionacearum Seaver, Mycologia 1(2): 62. 1909.
Synonym: Fusarium gigas Speg., Anal. Soc. cient. argent. 22(4): 221. 1886.
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Nectriaceae.
DNA barcodes: Acl = HQ897912, actA = KM231233, BenA = KM232096, CaM = KM231411, his3 = KM231561, ITS = HQ897826, LSU = MH875086, RPB1 = KM232254, tef1 (part 1) = KM231958.
Typus: USA, Missouri, Lebanon, on living Lespedeza with Parodiella perisporioides, Jul. 1887, Kellerman 1003 [lectotype NY designated by Samuels et al. (1991)]; Florida, Tampa, near Hillsborough River State Park, on pyrenomycete on Fabaceae, Dec. 2006, T. Gräfenhan 2007-03 (epitype designated here, CBS 125495, MBT 10020759, preserved as metabolically inactive culture, culture ex-epitype CBS 125495 = DAOM 238119).
Notes: The epitype designated here matches the morphology of Fusarium gigas (sensu Wollenweber 1916), and the lectotype of Nectria papilionacearum ( Samuels et al. 1991). This studied culture was originally listed by Gräfenhan et al. (2011), and we choose to now designate CBS 125495 as epitype to fix the application of the name.
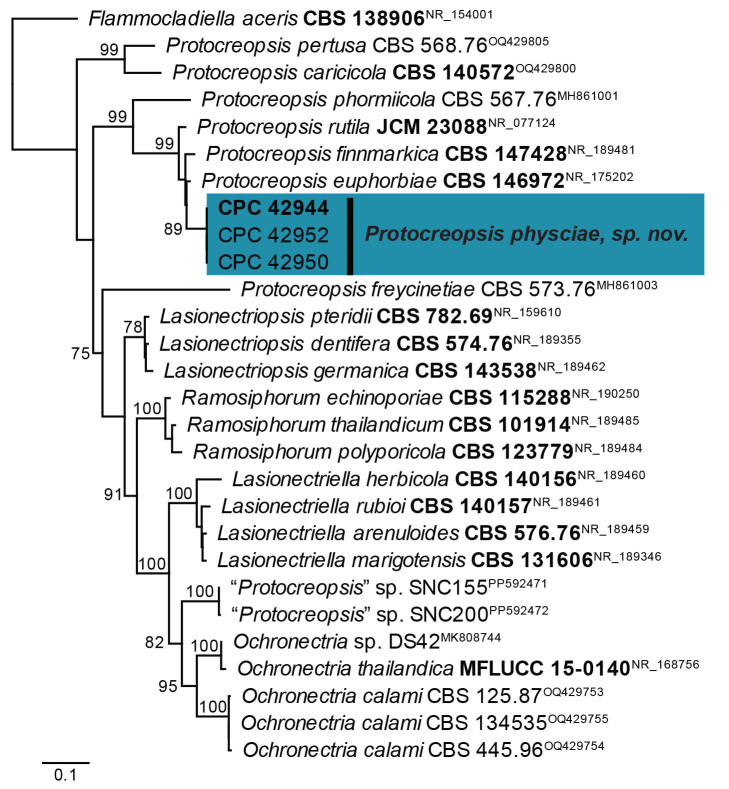
Macroconia podocarpi Crous & Sand.-Den., sp. nov. MycoBank MB 854316. Figs 24, 26
Fig. 26.
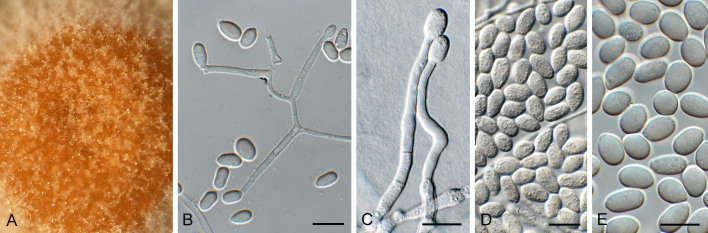
Macroconia podocarpi (CPC 47691). A. Conidia on twig in vivo. B–D. Conidia forming on SNA surface. E–G. Conidiophores and conidiogenous cells giving rise to conidia. H–J. Chlamydospore-like cells forming inside conidia. K. Conidia (note germination from apical or subapical cell). Scale bars: A–D = 20 μm; E–K = 10 μm.
Etymology: Name refers to the host genus it was isolated from, Podocarpus.
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Nectriaceae.
DNA barcodes: Acl = PP620545, BenA = PP620573, CaM = PP620549, ITS = PP590166, LSU = PP590170, RPB1 = PP620555, RPB2 = PP620563.
Description (7 d): Mycophilic, occurring on ascomycetous fungi, corticolous. Sporodochia pale orange, formed on agar surface and on carnation leaves. Sporodochial conidiophores simple to laterally branched, 15–80 × 4–5 μm, bearing terminal monophialides. Sporodochial conidiogenous cells monophialidic, subcylindrical, 12–40 × 4–5 μm, smooth, thin-walled, with apical collarette, in rare cases with percurrent elongation of phialide. Sporodochial conidia falcate, gently dorsiventrally curved, with elongate, blunt, slightly curved apical cell; basal cell well-developed, foot-shaped, (11–)15–18(–20)-septate, (135–)150–180(–200) × (13–)14–15 μm. Chlamydospores absent, but older conidia developing chlamydospore-like cells inside conidium. Sexual morph not observed in culture or on host of this specimen, but present on the epitype specimen. Nectria-like sexual morph poorly developed, but also present on specimen.
Culture characteristics: Colonies flat, spreading with moderate aerial mycelium and smooth, lobate margins, reaching 30 mm diam after 7 d. On MEA surface and reverse saffron; on PDA surface and reverse buff; on OA surface buff, with age turning orange.
Typus: South Africa, Eastern Cape Province, Haga Haga, Amathole, on ascomata of ascomycete on twigs of Podocarpus falcatus, Dec. 2022, M.J. Wingfield, HPC 4352 (holotype CBS H-25371, culture ex-type CPC 47691= CBS 151611), culture CPC 47692.
Notes: Species of Macroconia mostly grow on stromata of other ascomycetes on herbaceous plants or deciduous trees ( Gräfenhan et al. 2011). The present collection matches the morphology of Fusarium gigas ( Gerlach & Nirenberg 1982), the presumed asexual morph of Macroconia papilionacearum ( Gräfenhan et al. 2011), with reference strain CBS 125495 = DAOM 238119. The discussion of Gerlach & Nirenberg (1982) summarises observations of Wollenweber, Joffe and Booth, and suggests that F. gigas probably represents a species complex (based on differences in growth rate in culture, conidial dimensions and septation). Wollenweber (1916) cites conidia of F. gigas to be 9–12-septate, 100–130 × 7–13 μm, thus smaller and with less septa than observed in M. podocarpi. Unfortunately, only a few perithecia of M. podocarpi were observed, and thus no description of the sexual morph could be determined.
Authors: P.W. Crous & M. Sandoval-Denis
Neosatchmopsis Crous, M.A. Delgado & R.K. Schumach., gen. nov. MycoBank MB 854317.
Etymology: Name refers to its morphological similarity to the genus Satchmopsis.
Classification: Leotiomycetes, Leotiomycetidae, Helotiales, incertae sedis.
DNA barcodes: ITS = PP872398, LSU = PP872409.
Description: Sporodochia erumpent on leaves in vivo, drying down and collapsing in centre, appearing cup-like; conidiomata resembling Satchmopsis, but conidiomata lack lateral walls and are sporodochial, arising from a central stroma which is brown, but conidiophores and conidia remain hyaline. Conidiophores hyaline, smooth, subcylindrical, branched, septate. Conidiogenous cells hyaline, smooth, subcylindrical, phialidic, terminal and intercalary. Conidia solitary, aseptate, guttulate, hyaline, smooth, curved, subcylindrical, ends subobtuse.
Type species: Neosatchmopsis ogrovei Crous, M.A. Delgado & R.K. Schumach.
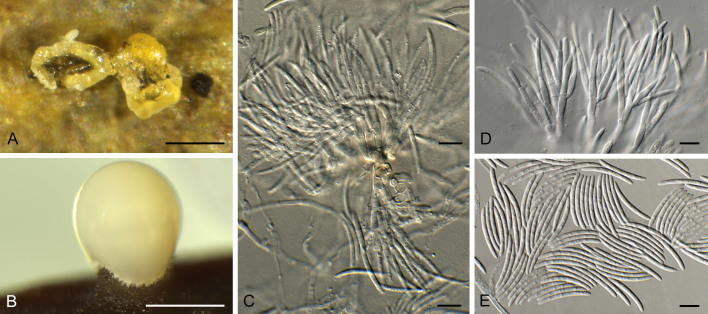
Neosatchmopsis ogrovei Crous, M.A. Delgado & R.K. Schumach., sp. nov. MycoBank MB 854319. Figs 27, 28.
Fig. 27.
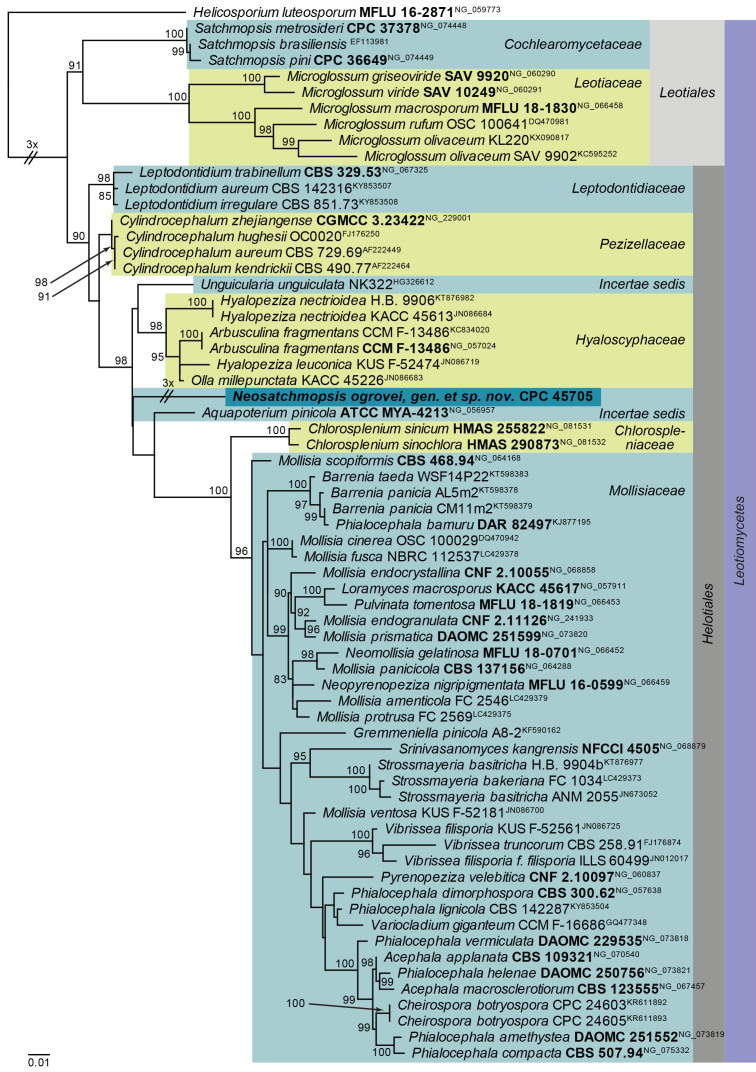
Maximum Likelihood (ML) phylogeny of Neosatchmopsis and allied genera based on the LSU nucleotide alignment. The dataset was aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and the ML tree was calculated in IQ-TREE v. 2.1.3 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. The tree was rooted to Helicosporium luteosporum (MFLU 16-2871, GenBank NG_059773). Values at nodes are ML ultrafast bootstrap ≥ 75 % (based on 10 000 replicates; values ≥ 95 % can be considered significant). The novel taxon is shown in bold and a dark blue block, and sequences from material with a type status are indicated with a bold culture or voucher number. Families, orders and the class are shown in coloured blocks on the right side of the phylogeny. Some branches were shortened to facilitate layout.
Fig. 28.
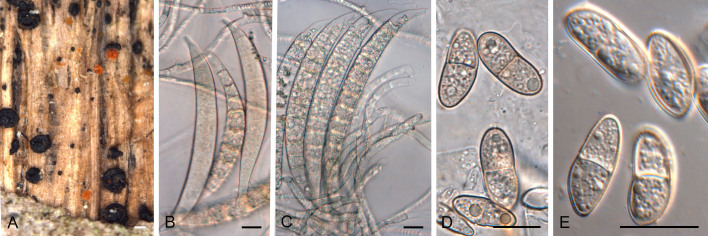
Neosatchmopsis ogrovei (CPC 45705). A. Erumpent sporodochia on leaves. B. Sporodochium giving rise to mucoid conidial mass on pine needle. C, D. Conidiophores and conidiogenous cells giving rise to conidia. E. Conidia. Scale bars: A, B = 350 μm; C–E = 10 μm.
Etymology: Based on the region of O Grove, where the specimen was collected.
Description (14 d): Sporodochia erumpent on leaves in vivo, drying down and collapsing in centre, appearing cup-like; conidiomata up to 350 μm diam, resembling Satchmopsis, but conidiomata lack lateral walls and are sporodochial, arising from a central stroma which is brown, but conidiophores and conidia remain hyaline. Conidiophores hyaline, smooth, subcylindrical, branched, up to 60 μm tall, 3–4 μm wide, 2–6-septate. Conidiogenous cells hyaline, smooth, subcylindrical, phialidic, 6–12 × 2–2.5 μm, terminal and intercalary. Conidia solitary, aseptate, guttulate, hyaline, smooth, curved, subcylindrical, ends subobtuse, (17–)18–19(–20) × 2 μm (in vitro), 19.6–30.2 × 1.2–2.2 μm (in vivo).
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium and smooth, even margin, reaching 20 mm diam after 2 wk at 25 °C. On MEA surface peach, outer region saffron and reverse ochreous; on PDA surface and reverse umber, outer region cream; on OA surface saffron.
Typus: Spain, Pontevedra, O Grove, on dead fallen leaf of Eucalyptus sp. (Myrtaceae), 13 Feb. 2023, M.A. Delgado, HPC 4146 = RKS 1181 (holotype CBS H-25326, culture ex-type CPC 45705 = CBS 150890).
Notes: When viewed with the dissecting microscope the present fungus appears to have cupulate conidiomata, resembling that of Satchmopsis (based on S. brasiliensis, on Eucalyptus; Crous et al. 2006). Upon closer inspection however, the conidiomata lack lateral walls and are actually sporodochia with a central stroma. The conidiogenous cells are phialidic, and the conidia are subcylindrical and aseptate, again resembling Satchmopsis ( Crous et al. 2020b), from which it is phylogenetically distinct.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Chaetomellaceae sp. [strain KL403, GenBank LT158476.1; Identities = 447/448 (99 %), no gaps], and distant hits with Pilidium septatum [voucher BCC 79016, GenBank NR_156616.1; Identities = 271/304 (89 %), 16 gaps (5 %)], Miricatena prunicola [strain CBS 149448, GenBank OQ990115.1; Identities = 205/210 (98 %), four gaps (1 %)], and Cadophora fastigiata [strain fung12, GenBank MT635284.1; Identities = 204/210 (97 %), four gaps (1 %)]. Closest hits using the LSU sequence are Arbusculina irregularis [strain VG76-8, GenBank OM906795.1; Identities = 800/863 (93 %), seven gaps (0 %)], Aquapoterium pinicola [strain ATCC MYA-4213, GenBank NG_056957.1; Identities = 814/879 (93 %), seven gaps (0 %)], and Hyaloscypha epiporia [strain CBS 125.91, GenBank MH873924.1; Identities = 813/879 (92 %), seven gaps (0 %)].
Authors: P.W. Crous, J.Z. Groenewald, M.A. Delgado & R.K. Schumacher
Ophiocordyceps kuchinaraiensis Khons., Thanakitp. & Luangsaard, sp. nov. MycoBank MB 854324.
[originally published as Ophiocordyceps kuchinaraiensis Khons. et al., Persoonia 50: 297. 2023. Nom. inval., Art. 40.1 (Shenzhen).
Etymology: Refers to the place where the type specimen was found, Khok Pa Si community forest, Kuchinarai District, Kalasin Province, Thailand.
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Ophiocordycipitaceae.
DNA barcodes: ITS = OQ627396, LSU = OQ627397, tef1 = OQ625474, RPB2 = OQ625475.
Description and illustration: Crous et al. (2023: 297).
Typus: Thailand, Kalasin Province, Kuchinarai District, Khok Pa Si Community Forest, on Coleoptera larva, buried in soil, 8 Jun. 2021, A. Khonsanit, D. Thanakitpipattana & K. Tasanathai (holotype BBH 50310, culture ex-type BCC 95830).
Notes: Ophiocordyceps kuchinaraiensis was invalidly published ( Crous et al. 2023), as the holotype specimen was a culture, which was not preserved in a metabolically inactive state. The name is validated here with a dried fungarium specimen to serve as holotype.
Authors: A. Khonsanit, D. Thanakitpipattana & J.J. Luangsa-ard
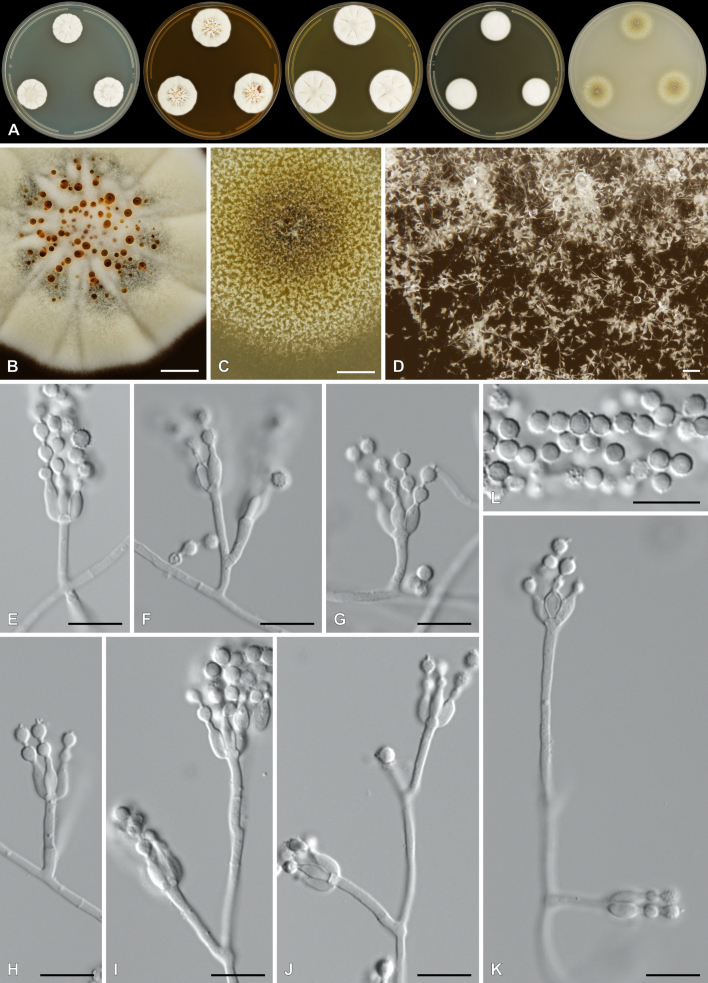
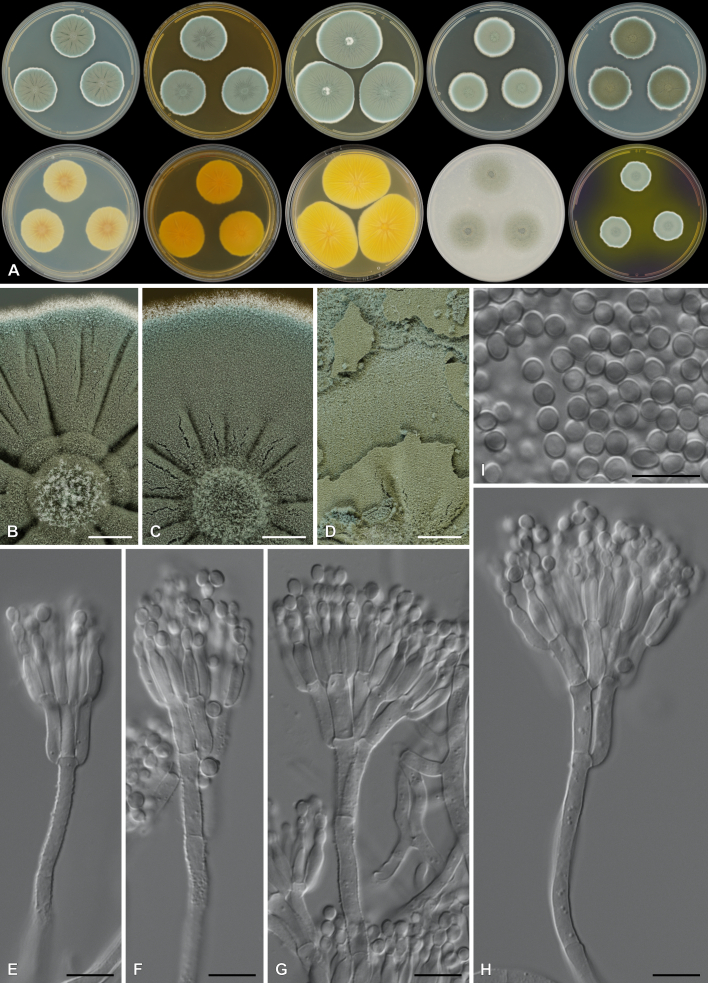
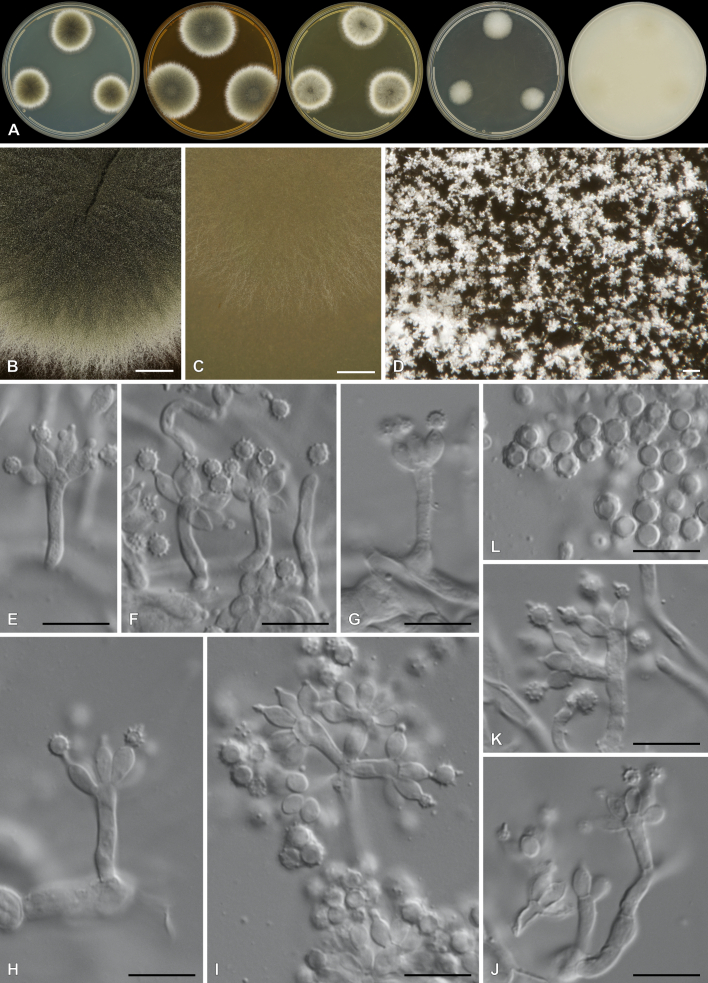
Penicillium cederbergense Visagie, Dewing & van Vuuren, sp. nov. MycoBank MB 854284. Figs 29, 30.
Fig. 29.
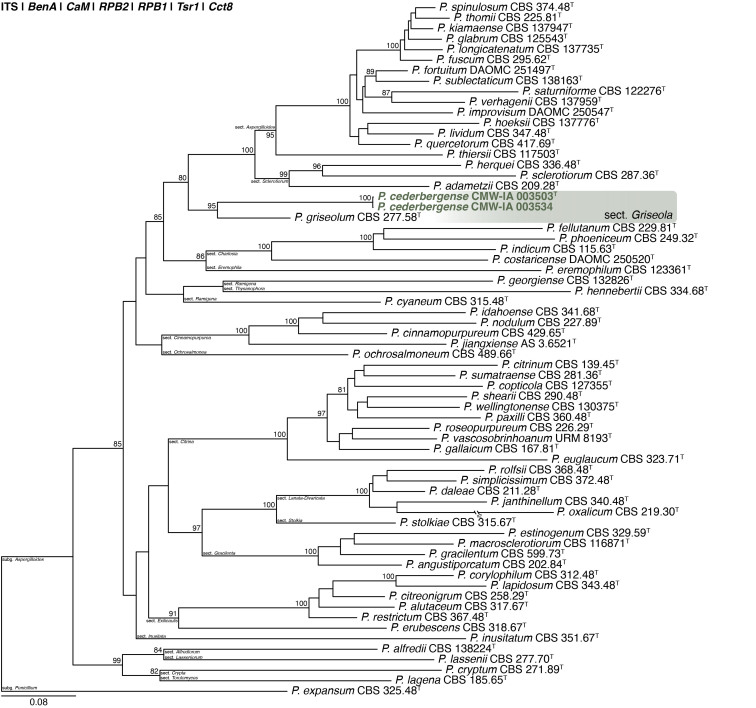
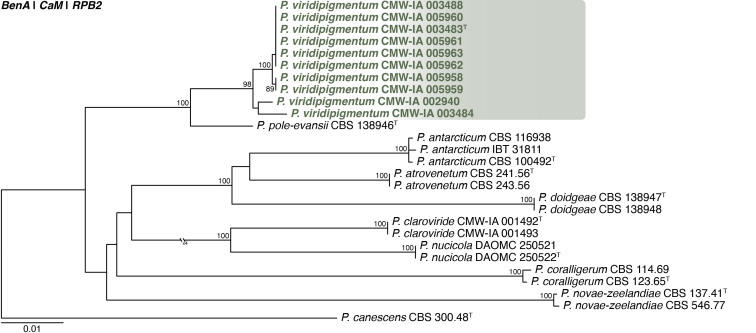
Multigene phylogeny of Penicillium subg. Aspergilloides based on ITS, BenA, CaM, RPB1, RPB2, Cct8 and Tsr1 sequences (see Suppl. Table S8). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Each region was treated as separate partitions, also taking into consideration introns and exons, and the most appropriate nucleotide substitution model based on the Akaike information criterion was applied to each using PartitionFinder v. 2 ( Lanfear et al. 2017). The tree was rooted to P. expansum. Penicillium cederbergense strains are shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Fig. 30.
Penicillium cederbergense. A. Colonies (left to right) on CYA, MEA, YES, DG18 and OA. B–D. Close-up of colonies on MEA (B), OA (C) and MEA (D). E–K. Conidiophores. L. Conidia. Scale bars: B, C = 2 mm; D = 100 μm; E–L = 10 μm.
Etymology: Latin, cederbergense, named after the Cederberg mountains from where the species was isolated.
Classification: Ascomycota, Eurotiomycetes, Eurotiales, Aspergillaceae, Penicillium, subgenus Aspergilloides, section Griseola, series Griseola.
DNA barcodes: ITS = PP375125, BenA = PP356422, CaM = PP356450, RPB2 = PP356483, RPB1 = PP356514, Cct8 = PP356534, Tsr1 = PP356554. Colony diam (in mm, after 7 d, at 25 °C): CYA 19–20; CYA 10 °C 4–6; CYA 15 °C 11–13; CYA 20 °C 19–22; CYA 30 °C 10–11; CYA 37 °C no growth; CYAS 11–13; MEA 26–29; DG18 20–22; YES 26–30; OA 22–26; CREA 7–12.
Colony characters (25 °C, 7 d): Colonies on CYA moderately deep, radially and slightly concentrically sulcate, slightly raised at centre; margins low, narrow, entire; mycelia white; texture floccose; sporulation absent to very sparse, conidia en masse not determined; soluble pigments absent; exudates minute clear droplets; reverse Pale to Greyish Yellow (4A3–B4). Colonies on MEA deep, radially sulcate; margins low, narrow, entire; mycelia white to olive, inconspicuous; texture floccose; sporulation sparse, conidia en masse Dull Green (26D3); soluble pigments absent; exudates brownish orange; reverse Brownish Yellow (5C7–8). Colonies on YES moderately deep, radially sulcate, slightly raised at centre; margins low, narrow, entire; mycelia white to cream; texture floccose; sporulation absent, conidia en masse not determined; soluble pigments absent; exudates minute clear droplets; reverse Light to Orange Yellow (3A4–4A4–6). Colonies on DG18 moderately deep, lightly sulcate; margins low, narrow, entire; mycelia white to olive, inconspicuous; texture floccose; sporulation very sparse, conidia en masse Greyish Green (25B3); soluble pigments absent; exudates minute clear droplets; reverse Yellowish White to Light Yellow (2A2–3A5), sometimes Yellow (3A8) at centre. Colonies on OA low, plane; margins low, wide, entire; mycelia white; texture velutinous to floccose; sporulation moderately dense, conidia en masse Dull Yellow to Olive (3B4–3E5); soluble pigments absent; exudates minute clear droplets. Colonies on CREA weak growth, acid production absent.
Conidiophores monoverticillate, might sometimes appear somewhat divaricate; stipes smooth, 10–100 × 1.5–2.5 μm; vesicles 2.5–3.5(–4) μm; phialides ampulliform, 3–5 per stipe, 6–7.5 × 2–3 μm (6.6 ± 0.5 × 2.6 ± 0.2); conidia in long chains with distinct connectors, rough to spiny, globose, 3–3.5 × 3–3.5 μm (3.2 ± 0.2 × 3.1 ± 0.1), average width/length = 0.96, n = 50.
Typus: South Africa, Western Cape Province, Cederberg, Middelberg
Waterfall (−32.365095, 19.069887), from soil, 28 Jun. 2020, coll. M.J. Wingfield, isol. C.M. Visagie [holotype PRU(M) 4582, culture ex-type CMW-IA 003503 = CMW 61469 = CBS 152021 = CN072G2].
Additional material examined: South Africa, Western Cape Province, Cederberg, Middelberg Waterfall (−32.365095, 19.069887), from soil, 28 Jun. 2020, coll. M.J. Wingfield, isol. C. Dewing, culture CMW-IA 003534 = CMW 61497 = CBS 152022 = CN077E1 = CN164F4.
Notes: A multigene phylogeny resolves P. cederbergense as the closest relative of P. griseolum (Fig. 29 ), the only species currently classified in sect. Griseola ( Houbraken et al. 2020). Similarities between P. cederbergense and P. griseolum are low with ITS differing by at least 26 bp, BenA by at least 69 bp, CaM by at least 108 bp, RPB1 by at least 123 bp, RPB2 by at least 89 bp, Cct8 by at least 57 bp and Tsr1 by at least 87 bp. The new species could represent a new series in the section, but additional strains, data and analyses are needed to confirm this. No modern description is available for P. griseolum, but this species is reported to produce funicles on MEA ( Smith 1957), compared to the floccose texture of P. cederbergense. Their micromorphologies are largely similar, both species characterised by short monoverticillate conidiophores that produce globose conidia with rough to spiny walls ( Smith 1957).
Authors: C.M. Visagie, C. Dewing & N.I. van Vuuren
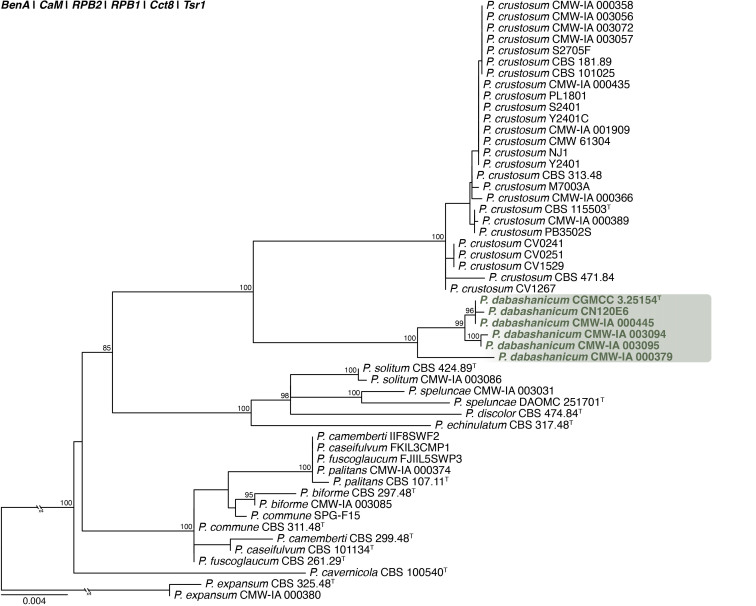
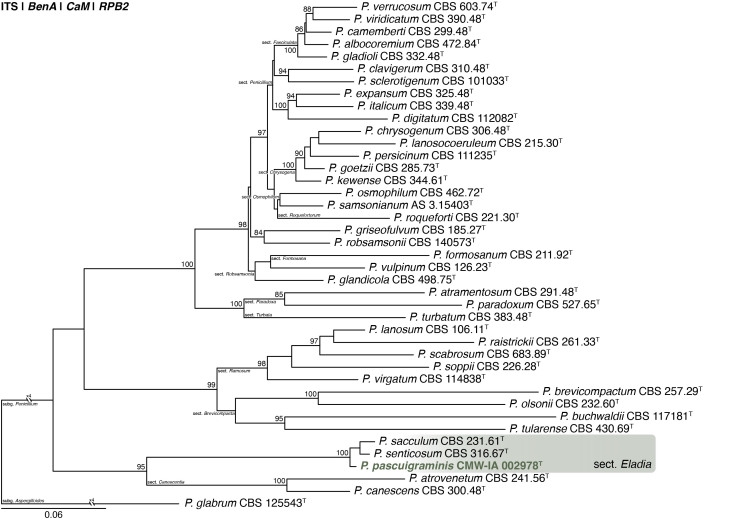
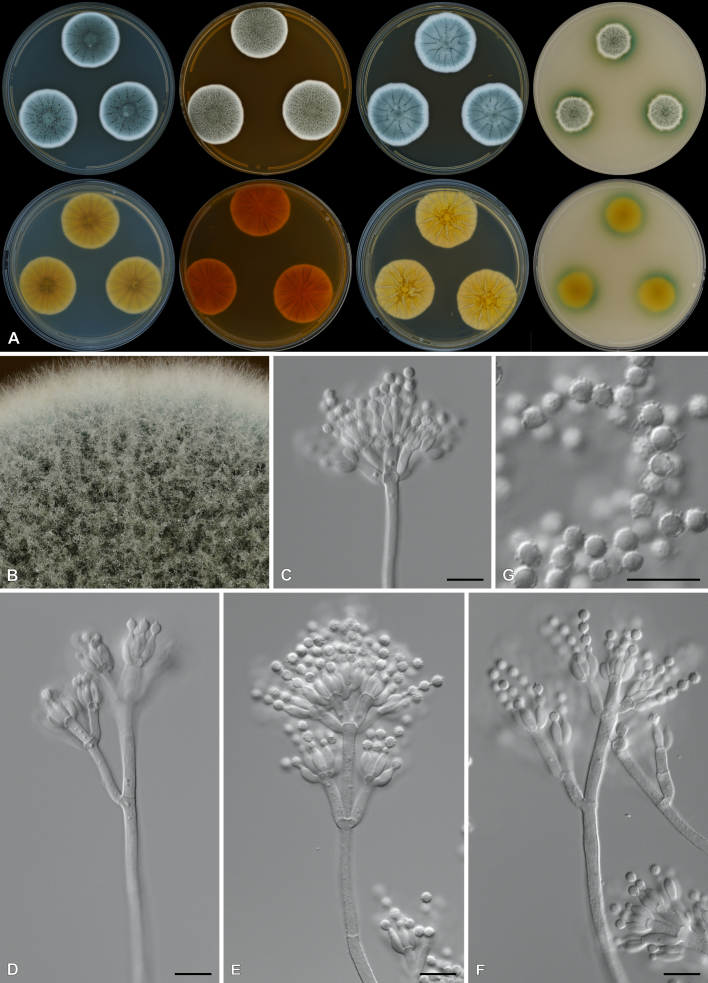
Penicillium dabashanicum X.C. Wang & W.Y. Zhuang, J. Fungi 9(12, no. 1150): 65. 2023. Figs 31, 32 . .
Fig. 31.
Multigene phylogeny of Penicillium sect. Fasciculata ser. Camembertiorum based on BenA, CaM, RPB1, RPB2, Cct8 and Tsr1 sequences (see Suppl. Table S9). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Each region was treated as separate partitions, also taking into consideration introns and exons, and the most appropriate nucleotide substitution model based on the Akaike information criterion was applied to each using PartitionFinder v. 2 ( Lanfear et al. 2017). The tree was rooted to P. expansum. Penicillium dabashanicum strains are shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Fig. 32.
Penicillium dabashanicum. A. Colonies (top row, left to right) on CYA, MEA, YES, DG18 and CYAS; (bottom row, left to right) CYA reverse, MEA reverse, YES reverse, OA, CREA. B–D. Close-up of colonies on CYA (B), MEA (C) and MEA after 14 d showing conidia breaking off in crusts (D). E–H. Conidiophores. I. Conidia. Scale bars: B–D = 2 mm; E–I = 10 μm.
Classification: Ascomycota, Eurotiomycetes, Eurotiales, Aspergillaceae, Penicillium, subgenus Penicillium, section Fasciculata, series Camembertiorum.
DNA barcodes: ITS = OQ870786, BenA = OR051047, CaM = OR051226, RPB2 = OR051400.
Colony diam (in mm, after 7 d, at 25 °C): CYA 28–32; CYA 10 °C 8–13; CYA 15°C 22–26; CYA 20 °C 33–35; CYA 30 °C 15–18; CYA 37 °C no growth; CYAS 29–34; MEA 29–32; DG18 25–27; YES 40–42; OA 22–23; CREA 19–24.
Colony characters (25 °C, 7 d): Colonies on CYA moderately deep, radially sulcate, slightly crateriform; margins low, narrow, entire; mycelia white; texture velutinous, slightly fasciculate to crustose; sporulation dense, conidia en masse Dull to Greyish Green (25D4–5); soluble pigments absent; exudates minute droplets, clear; reverse Light Brown (5D5), Greyish Green (30B3). Colonies on MEA moderately deep, radially sulcate, slightly crateriform; margins low, narrow, entire; mycelia white; texture velutinous to crustose; sporulation dense, conidia en masse Greyish Green (25D5–E5); soluble pigments absent; exudates absent; reverse Olive Brown (4D6), Light Brown (5D7). Colonies on YES moderately deep, radially sulcate, crateriform; margins low, narrow, entire; mycelia white; texture velutinous to crustose; sporulation dense, conidia en masse Dull to Greyish Green (25D4–5); soluble pigments absent; exudates absent; reverse Greyish Yellow (2B5), Yellowish Orange (4B7). Colonies on DG18 moderately deep, plane, slightly crateriform; margins low, narrow, entire; mycelia white, inconspicuously yellow; texture velutinous, slightly fasciculate; sporulation dense, conidia en masse Dull to Greyish Green (25D4–5); soluble pigments absent; exudates absent; reverse Greyish Yellow (1B6), Greyish Green (1C6–7). Colonies on OA low, plane; margins low, wide, entire; mycelia white; texture velutinous, slightly fasciculate; sporulation dense, conidia en masse Greyish Green (25D5–E6–26E5); soluble pigments absent; exudates clear. Colonies on CREA strong growth, acid produced.
Conidiophores terverticillate, sometimes biverticillate; stipes rough, 100–300 × 3.5–4.5 μm; branches 2 per stipe, 13.5–27 × 3.5–4.5 μm; metulae 4–5 per branch, 11.5–16(–19) × 3.5–5 μm; phialides ampulliform, 4–6 per metula, 9.5–12 × 3–4(–4.5) μm (10.7 ± 0.7 × 3.5 ± 0.3); average length metula/phialide 1.3; conidia smooth, subglobose to broadly ellipsoid, 3.5–4(–4.5) × 3–3.5(–4) μm (3.9 ± 0.2 × 3.3 ± 0.2), average width/length = 0.85, n = 53.
Typus: China, Chongqing City, Chengkou County, Daba Mountain National Nature Reserve, Gaoguan Town, at the riverside of Ren River, in soil, 30 Oct. 2020, X. Wang, H. Zheng & C. Liu (holotype HMAS 247893, culture ex-type CGMCC 3.25154).
Additional materials examined: South Africa, North West Province, Rostrataville (−26.79312, 25.69841), from pre-stored maize, 16 Jul. 2020, C.M. Visagie, culture CMW-IA 000379 = CMW 59900 = CBS 152013 = CN054H1; from pig feed, 21 Mar. 2021, C.M. Visagie, culture CMW-IA 000445 = CMW 59966 = CBS 152014 = CN138A9; Eastern Cape, Cofimvaba (−31.96978997, 27.66315403), from maize, 14 Jul. 2020, J. Price, cultures CMW-IA 003094 = CMW 61308 = CBS 152019 = CN045B9, and CMW-IA 003095 = CMW 61309 = CBS 152020 = CN045C4; from cattle feed, 21 Mar. 2021, A. Hobden, culture CN120E6.
Notes: Penicillium dabashanicum was recently described as the closest relative of P. crustosum ( Wang et al. 2023). Additional P. dabashanicum strains were isolated during the last few years in South Africa from sources such as maize, maize field soils and animal feed. Morphologically, P. dabashanicum and P. crustosum are almost identical. However, P. dabashanicum grows slightly slower on CYA (28–32 vs 32–46 mm) and OA (22–23 vs 28–32 mm), its conidia is generally larger [3.5–4(–4.5) × 3–3.5(–4) vs 3–4 μm] and is subglobose to broadly ellipsoid vs globose to subglobose ( Frisvad & Samson 2004). We note that P. dabashanicum was described as having smooth-walled stipes but they are consistently roughened in our strains, which is similar to P. crustosum. Phylogenetically they are distinct species with phylogenies consistently resolving strains in two clades (Fig. 31, Suppl Fig. 1). Similarities between P. dabashanicum and P. crustosum is low with BenA having at least 11 bp differences, CaM at least 10 bp differences, RPB1 at least 23 bp differences, RPB2 at least 19 bp differences, Cct8 at least 12 bp differences and Tsr1 at least 32 bp differences. They do, however, share identical ITS sequences.
Authors: C.M. Visagie, N. Yilmaz, N.I. van Vuuren, A. Hobden, J.-L. Price & A. Pringle
Penicillium pascuigraminis Visagie, Dewing & van Vuuren, sp. nov. MycoBank MB 854285. Figs 33, 34.
Fig. 33.
Multigene phylogeny of Penicillium subg. Penicillium based on ITS, BenA, CaM and RPB2 sequences (see Suppl. Table S10). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Each region was treated as separate partitions, also taking into consideration introns and exons, and the nucleotide substitution model GTR+I+G was applied to each ( Abadi et al. 2019). The tree was rooted to P. glabrum. Penicillium pascuigraminis is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Fig. 34.
Penicillium pascuigraminis. A. Colonies (left to right) on CYA, MEA, YES, DG18 and OA. B–D. Close-up of colonies on MEA (B), OA (C) and MEA (D). E–K. Conidiophores. L. Conidia. Scale bars: B, C = 2 mm; D = 100 μm; E–L = 10 μm.
Etymology: Latin, pascuigraminis, “pascuum” meaning pasture and “graminis” meaning grasses, named after the substrate this species was isolated from.
Classification: Ascomycota, Eurotiomycetes, Eurotiales, Aspergillaceae, Penicillium, subgenus Penicillium, section Eladia, series Eladia.
DNA barcodes: ITS = PP375131, BenA = PP356429, CaM = PP356452, RPB2 = PP356485.
Colony diam (in mm, after 7 d, at 25 °C): CYA 27–30; CYA 10 °C no growth; CYA 15 °C no growth; CYA 20 °C 17–20; CYA 30 °C 26–27; CYA 37 °C 20–21; CYAS 5–8; MEA 39–40; DG18 15–18; YES 29–32; OA 35–38; CREA 3–5.
Colony characters (25 °C, 7 d): Colonies on CYA low, plane; margins low, wide, entire; mycelia white; texture velutinous, some floccose regions centrally; sporulation moderately dense, conidia en masse Olive (1E5–F8); soluble pigments absent; exudates absent; reverse Yellowish White (2A2) to Greyish Green (1D3–4). Colonies on MEA low, plane; margins low, wide, entire; mycelia white; texture velutinous, some floccose regions centrally; sporulation moderately dense, conidia en masse Olive (1E5–F8); soluble pigments absent; exudates absent; reverse Olive brown (4D8–F8). Colonies on YES low, plane; margins low, wide, entire; mycelia white; texture velutinous, some floccose regions centrally; sporulation moderately dense, conidia en masse Olive (1E5–F8); soluble pigments absent; exudates absent; reverse Olive Brown (4D6). Colonies on DG18 low, plane; margins low, wide, entire; mycelia white; texture yeast-like, leathery; sporulation absent, conidia en masse not determined; soluble pigments absent; exudates absent; reverse Yellowish white (2A2). Colonies on OA low, plane, sparsely spread; margins low, wide, entire; mycelia white; texture velutinous; sporulation sparse to moderately dense, conidia en masse olive, inconspicuous; soluble pigments absent; exudates absent. Colonies on CREA weak growth, acid production absent.
Conidiophores monoverticillate, small proportion with subterminal branches; stipes smooth, (5–)9–15(–23) × 2–3 μm; vesicle 3.5–4(–5) μm; phialides ampulliform, 3–7 per stipe, 5–6.5(–7) × 2.5–4 μm (5.8 ± 0.5 × 3.06 ± 0.3); conidia in short chains with distinct connectors, echinulate, globose, 3–3.5 × 3–3.5 μm (3.2 ± 0.26 × 3.2 ± 0.22), average width/length = 0.98, n = 47.
Typus: South Africa, Eastern Cape Province, Humansdorp (−34.003599, 24.74712), from pasture mulch, May 2020, coll. A. Davis, isol. C. Dewing [holotype PRU(M) 4581, culture ex-type CMW-IA 002978 = CMW 61192 = CN049E3].
Notes: Penicillium pascuigraminis belongs to Penicillium sect. Eladia and is a close relative of P. sacculum and P. senticosum, the only other species in the section (Fig. 33, Suppl. Fig. 2).
Both species are only known from ex-type material. Penicillium pascuigraminis, differs from P. sacculum and P. senticosum by at least 2 bp based on ITS, 9 bp based on BenA, 8 bp based on CaM and 4 bp based on RPB2. Morphologically, the new species shows similar characters as its closest relatives, producing moderately fast-growing colonies, with velutinous texture and olive-coloured conidia, while conidiophores are short, monoverticillate that produce thick walled, spiny, globose conidia in short chains with distinct connectors ( Smith 1961, Pitt 1980, Houbraken & Samson 2011, Houbraken et al. 2020). Penicillium pascuigraminis can be distinguished from P. sacculum based on its faster growth on CYA (27–30 vs 14–15 mm) and its smaller conidia (3–3.5 vs 5–6 μm) ( Smith 1961, Houbraken & Samson 2011). Compared to P. senticosum, the new species generally grows faster on CYA (27–30 vs 10–30 mm) and CYA at 37 °C (20–21 vs 2–12 mm) and also did not produce a sexual morph, unlike its close relative ( Pitt 1980).
Authors: C.M. Visagie, C. Dewing & N.I. van Vuuren
Penicillium viridipigmentum van Vuuren, Mthembu, Dewing, Qikani, Yilmaz & Visagie, sp. nov. MycoBank MB 854286. Figs 35, 36.
Fig. 35.
Multigene phylogeny of Penicillium sect. Canescentia ser. Atroveneta based on BenA, CaM and RPB2 sequences (see Suppl. Table S11). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Each region was treated as separate partitions, also taking into consideration introns and exons, and the nucleotide substitution model GTR+I+G was applied to each ( Abadi et al. 2019). The tree was rooted to P. canescens. Penicillium viridipigmentum is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Fig. 36.
Penicillium viridipigmentum. A. Colonies (top, left to right) on CYA, MEA, YES and OA (colony reverses in bottom row). B. Close-up of colony on MEA. C–F. Conidiophores. G. Conidia. Scale bars: C–G = 10 μm.
Etymology: Latin, viridipigmentum, “viridis” meaning green and “pigmentum” meaning pigments, named after the striking green pigment produced by this species on especially oatmeal agar.
Classification: Ascomycota, Eurotiomycetes, Eurotiales, Aspergillaceae, Penicillium, subgenus Penicillium, section Canescentia, series Atroveneta.
DNA barcodes: ITS = PP375132, BenA = PP356427, CaM = PP356431, RPB2 = PP356464.
Colony diam (in mm, after 7 d, at 25 °C): CYA 24–33; CYA 10 °C 5–7; CYA 15 °C 13–19; CYA 20°C 21–25; CYA 30 °C 20–27; CYA 37 °C no growth; CYAS 26–34; MEA 25–30; DG18 24–33; YES 33–38; OA 18–27; CREA 8–10.
Colony characters (25 °C, 7 d): Colonies on CYA moderately deep, radially sulcate, slightly crateriform; margins low to moderate, narrow, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse Dull to Greyish Green (25D4–26D3–27C4); soluble pigments present, yellow; exudates yellow; reverse Olive Yellow (3D6), Yellowish Brown (5D6). Colonies on MEA moderately deep, lightly sulcate, raised at centre; margins low to moderate, narrow, entire; mycelia white; texture floccose; sporulation moderate to dense, conidia en masse Dull to Greyish Green (25D4–27D4–D5); soluble pigments absent; exudates yellow; reverse Yellowish Brown (5D6). Colonies on YES deep, radially sulcate, crateriform; margins deep, wide, entire; mycelia white; texture floccose; sporulation dense, conidia en masse Dull to Greyish Green (25C4–D4); soluble pigments absent; exudates absent; reverse Greyish Yellow (4B6–C6). Colonies on DG18 low to moderate, slightly sulcate, slightly raised at centre; margins low, narrow, entire; mycelia white; texture floccose; sporulation dense, conidia en masse Dull Green (26D4–E4); soluble pigments absent; exudates absent; reverse Yellowish Orange (4B7) and Greyish Yellow (3B5). Colonies on OA low, plane; margins low, narrow, entire; mycelia white; texture floccose; sporulation moderately dense, conidia en masse Greenish Grey (26D2); soluble pigments green; exudates yellow. Colonies on CREA weak growth, no acid production.
Conidiophores biverticillate, sometimes terverticillate; stipes finely rough to rough, 43.5–465 × 2–6 μm; branches 8–32 × 2.5–4.5 μm; metulae divergent, 2–6 per stipe/branch, 7–17.5 × 2.5–4.5 μm; phialides ampulliform, 5.5–10 × 2.5–4 μm (7.5 ± 0.9 × 3 ± 0.3); average length metula/phialide 1.53; conidia rough to spiny, globose, 2.5–4 × 2.5–4 μm (3 ± 0.3 × 3 ± 0.3), average width/length = 1, n = 100.
Typus: South Africa, Free State Province, Vrede, from soil, 2020, N. Qikani [holotype PRU(M) 4579, culture ex-type CMW-IA 003483 = CMW 59679 = CN069H6].
Additional materials examined: South Africa, Eastern Cape Province, Humansdorp (−34.003599, 24.74712), from pasture mulch, May 2020, coll. A. Davis, isol. C. Dewing, cultures CMW-IA 002940 = CMW 61154 = CBS 152018 = CN049F8, and CMW-IA 003488 = CMW 61454 = CN071D4; Free State Province, Vrede, from soil, 2020, N. Qikani, culture CMW-IA 003484 = CMW 60007 = CN069I8; Eastern Cape Province, Aberdeen (−32.387997, 24.217217), from soil, 21 Apr. 2021, C.M. Visagie, cultures CMW-IA 005958 = CMW 61977 = CBS 152023 = CN165B3, and CMW-IA 005959 = CMW 61978 = CN165B4; Eastern Cape Province, Middelburg (−31.837003, 24.860625), from soil, 21 Apr. 2021, C.M. Visagie, cultures CMW-IA 005960 = CMW 61979 = CN165B5, CMW-IA 005961 = CMW 61980 = CN165B6, CMW-IA 005962 = CMW 61981 = CN165B7, and CMW-IA 005963 = CMW 61982 = CN165B8.
Notes: Penicillium viridipigmentum is resolved in Penicillium sect. Canescentia ser. Atroveneta as the closest relative of P. pole-evansii (Fig. 35, Suppl. Fig. 3). The new species differs from P. pole-evansii by at least 2 bp based on ITS, 6 bp based on BenA, 12 bp based on CaM and 16 bp based on RPB2. Penicillium viridipigmentum produce colonies with distinctive green soluble pigments on OA, a character also known for P. pole-evansii and P. nucicola ( Visagie et al. 2016). The colonies of P. nucicola grows more restricted than both these species (12–14 vs >25 mm). It is difficult to distinguish P. viridipigmentum from P. pole-evansii using morphological characters. However, the new species does produce slightly larger conidia (2.5–4 vs 2–3 μm) ( Visagie et al. 2021).
Authors: N.I. van Vuuren, A. Mthembu, C. Dewing, N. Qikani, N. Yilmaz & C.M. Visagie
Phragmotrichum platanoidis G.H. Otth, Mitt. naturf. Ges. Bern 723: 111. 1871. [1870] Figs 37, 38.
Fig. 37.
Maximum Likelihood (ML) phylogeny of Melanommataceae and allied families based on the LSU nucleotide alignment. The dataset was aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and the ML tree was calculated in IQ-TREE v. 2.1.3 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. The tree was rooted to Ceratosphaeria lampadophora (CPC 33633, GenBank MN317269). Values at nodes are ML ultrafast bootstrap ≥ 75 % (based on 10 000 replicates; values ≥ 95 % can be considered significant). The novel taxon is shown in bold and a dark blue block, and sequences from material with a type status are indicated with a bold culture or voucher number. Families, the order and the class are shown in coloured blocks on the right side of the phylogeny. The basal branch was shortened to facilitate layout.
Fig. 38.
Phragmotrichum platanoidis (CPC 45665). A. Colony on OA. B–D. Conidiophores and conidiogenous cells giving rise to conidia. E, F. Muriformly septate conidia, also occurring in short, unbranched chains. Scale bars: A = 250 μm; B–F = 10 μm.
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Melanommataceae.
DNA barcodes: ITS = PP872400, LSU = PP872411, RPB2 = PP874918.
Description (14 d): Conidiomata sporodochium-like, erumpent at maturity (in vivo), superficial in vitro, solitary, dark brown, 250–350 μm diam; wall composed of dark brown textura angularis. Conidiophores hyaline, branched at base, smooth, subcylindrical, arising from a basal stroma, 10–40 × 3–4 μm. Conidiogenous cells thallic, integrated, hyaline, smith, subcylindrical, giving rise to unbranched conidial chains, 10–17 × 3–4 μm. Conidia brown, muriformly septate, with 3–7 transverse and 1–3 longitudinal to oblique septa, truncate at base (3–4 μm diam), or at both ends, straight, fusoid to ellipsoid, smooth-walled, (14–)20–30(–40) × (6–)8–10(–11) μm.
Culture characteristics: Colonies erumpent, spreading, surface folded, with abundant aerial mycelium and smooth, lobate margin, reaching 45 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Switzerland, near Bern, on thin branches of Acer platanoides, unknown collection date and collector (holotype in BERN, isotype IMI 108390). Germany, Thuringia, Sonneberg, Spechtsbrunn, on dead fruit of Acer pseudoplatanus (Sapindaceae), 13 Jan. 2023, S. Heinz, HPC 4126 = herbar.nr. 1090 (epitype CBS H-25320 designated here MBT 10020765, culture ex-epitype CPC 45665 = CBS 150803).
Notes: Phragmotrichum is based on P. chailletii ( Crous et al. 2020a), to which P. platanoidis is closely related. An epitype is herewith proposed for P. platanoidis to fix the application of the name.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Alpinaria rhododendri [voucher WU 36914, GenBank NR_147686.1; Identities = 548/564 (97 %), two gaps (0 %)], Petrakia echinata [strain L55, GenBank KY189981.1; Identities = 546/570 (96 %), eight gaps (1 %)], and Herpotrichia juniperi [strain 442J, GenBank PP523956.1; Identities = 530/548 (97 %), one gap (0 %)]. The ITS sequence of Melanocucurbitaria uzbekistanica (voucher TASM 6109, GenBank NG_059865.1) is 94 % similar (473/504, including seven gaps) to that of CPC 45665. Closest hits using the LSU sequence are Melanocucurbitaria uzbekistanica [voucher TASM 6109, GenBank NG_059865.1; Identities = 858/870 (99 %), no gaps], Herpotrichia juniperi [strain Rac09Pj11-1, GenBank LC618845.1; Identities = 858/870 (99 %), no gaps], and Petrakia fagi [as Pseudodidymella fagi; strain LC203357, GenBank LC203357.1; Identities = 837/849 (99 %), no gaps]. Closest hits using the RPB2 sequence had highest similarity to Phragmotrichum thornhilliae [as Phragmotrichum sp. OK-2023a; strain NK468, GenBank OQ884627.1; Identities = 615/687 (90 %), no gaps], Phragmotrichum chailletii [strain NK462, GenBank OQ884631.1; Identities = 613/687 (89 %), no gaps], Seifertia azaleae [voucher ZTMyc_59954, GenBank MK502061.1; Identities = 604/687 (88 %), no gaps], and Petrakia deviata [voucher ZTMyc_57663, GenBank MK502056.1; Identities = 437/498 (88 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & T. Hülsewig
Pleurotheciella acericola Crous & T. Hülsewig, sp. nov. MycoBank MB 854322. Figs 39, 40.
Fig. 39.
Maximum Likelihood (ML) phylogeny based on the Pleurotheciella ITS nucleotide alignment. The dataset was aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and the ML tree was calculated in IQ-TREE v. 2.1.3 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. The tree was rooted to Conioscypha pleiomorpha (CBS 138110, GenBank NR_154659). Values at nodes are ML ultrafast bootstrap ≥ 75 % (based on 10 000 replicates; values ≥ 95 % can be considered significant). The novel taxon is shown in bold and a dark blue block, and sequences from material with a type status are indicated with a bold culture or voucher number.
Fig. 40.
Pleurotheciella acericola (CPC 45699). A. Colony on SNA. B–G. Conidiophores and conidiogenous cells giving rise to conidia. H. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus from which it was isolated, Acer.
Classification: Sordariomycetes, Hypocreomycetidae, Pleurotheciales, Pleurotheciaceae.
DNA barcodes: ITS = PP872401, LSU = PP872412, RPB2 = PP874919, tef1 (second part) = PP874925.
Description (14 d): Mycelium consisting of hyaline, smooth, branched, septate, 1.5–2 μm diam hyphae. Conidiophores arising from superficial hyphae, erect, subcylindrical, straight to geniculous-sinuous, hyaline, smooth, 1–2-septate, 10–25 × 3–4 μm. Conidiogenous cells integrated, terminal, 6–12 × 3–4 μm, with several apical denticles, subcylindrical, 1–3 × 1 μm; not thickened nor darkened. Conidia solitary, hyaline, smooth, guttulate, fusoid-ellipsoid to subcylindrical, slightly curved to straight, medianly 1-septate, apex subobtuse, tapering to truncate hilum, 0.5 μm diam, (12–)13–14(–17) × 3(–3.5) μm.
Culture characteristics: Colonies erumpent, spreading, surface folded, with moderate aerial mycelium and smooth, lobate margin, reaching 10 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Germany, North Rhine-Westphalia, Witten, recreation area Hohenstein, on stem, under bark of living tree of Acer sp. (Sapindaceae), 27 May 2022, T. Hülsewig, HPC 4135 = herbar.nr. 958 (holotype CBS H-25325, culture ex-type CPC 45699 = CBS 150809).
Notes: Pleurotheciella (based on P. rivularia) was established by Réblováblová et al. (2012) for species with chaetosphaeria-like ascomata and blastic conidia produced sympodially on a rachis or on denticles (dactyalaria-like asexual morphs). Pleurotheciella acericola is phylogenetically distinct from the species described to date ( Shi et al. 2021).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Pleurotheciella sp. [strain KUNCC 23-13753, GenBank OR589321.1; Identities = 524/533 (98 %), four gaps (0 %)], Pleurotheciella sp. WW-2023d [strain KUNCC 23-16569, GenBank PP049498.1; Identities = 517/535 (97 %), three gaps (0 %)], Pleurotheciella dimorphospora [strain KUMCC 20-0185, GenBank NR_175737.1; Identities = 550/572 (96 %), four gaps (0 %)], and Pleurotheciella saprophytica [voucher MFLU 17-0915, GenBank NR_160595.1; Identities = 480/502 (96 %), five gaps (0 %)]. Closest hits using the LSU sequence are Pleurotheciella dimorphospora [strain KUMCC 20-0185, GenBank NG_081519.1; Identities = 798/805 (99 %), no gaps], Pleurotheciella saprophytica [voucher MFLU 17-0915, GenBank NG_066196.1; Identities = 760/767 (99 %), no gaps], and Pleurotheciella fusiformis [strain MFLUCC 17-0115, GenBank MF399249.1; Identities = 698/726 (96 %), six gaps (0 %)]. Closest hits using the RPB2 sequence had highest similarity to Pleurotheciella dimorphospora [voucher MFLU 20-0138, GenBank MZ509665.1; Identities = 464/499 (93 %), no gaps], Pleurotheciella hyalospora [strain GZCC 22-2023, GenBank OP999220.1; Identities = 678/794 (85 %), three gaps (0 %)], and Pleurotheciella lunata [strain MFLUCC 17-0111, GenBank MF401407.1; Identities = 552/650 (85 %), three gaps (0 %)]. Closest hits using the tef1 (second part) sequence had highest similarity to Pleurotheciella dimorphospora [voucher MFLU 20-0138H, GenBank MZ509664.1; Identities = 796/825 (96 %), no gaps], Pleurotheciella sympodia [strain MFLUCC 15-0996, GenBank MZ005575.1; Identities = 783/832 (94 %), no gaps], and Dematipyriforma aquilariae [strain MFLUCC 17-2382, GenBank OP473035.1; Identities = 781/830 (94 %), no gaps].
Authors: P.W. Crous, J.Z. Groenewald & T. Hülsewig
Protocreopsis physciae Crous & Boers, sp. nov. MycoBank MB 854323. Figs 41, 42.
Fig. 41.
Maximum Likelihood (ML) phylogeny of Protocreopsis and allied genera based on the ITS nucleotide alignment. The dataset was aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and the ML tree was calculated in IQ-TREE v. 2.1.3 ( Minh et al. 2020). Best nucleotide substitution models were calculated with ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. The tree was rooted to Flammocladiella aceris (CBS 138906, GenBank NR_154001). Values at nodes are ML ultrafast bootstrap ≥ 75 % (based on 10 000 replicates; values ≥ 95 % can be considered significant). The novel taxon is shown in bold and a dark blue block, and sequences from material with a type status are indicated with a bold culture or voucher number.
Fig. 42.
Protocreopsis physciae (CPC 42944). A. Colony on OA. B, C. Conidiogenous cells giving rise to conidia. D, E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Physcia from which it was isolated.
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Bionectriaceae.
DNA barcodes: ITS = PP872402, LSU = PP872413, actA = PP874914, tef1 (first part) = PP874920, tub2 = PP874927.
Description (14 d): Conidiophores solitary, or aggregated in clusters, forming sporodochia with mucoid, orange spore masses; conidiophores branched, septate, subcylindrical, up to 100 μm tall, 3–4 μm diam. Conidiogenous cells integrated, terminal and intercalary, phialidic, hyaline, smooth, subcylindrical to aculeiform, flexuous or straight with slight apical taper, 2–2.5 μm diam at apex, with periclinal thickening and cylindrical collarette, 1–3 μm long, 20–40 × 3–4 μm. Conidia solitary, aseptate, hyaline, smooth, granular, ellipsoid, apex subobtuse, base truncate, 2–3 μm, (9–)11–13(–15) × (5–)5.5–6(–7) μm.
Culture characteristics: Colonies erumpent, spreading, with folded surface, sparse aerial mycelium and feathery, lobate margin, reaching 6 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse orange.
Typus: Netherlands, Friesland Province, Koehool, Zeedijk, on Physcia caesia (Physciaceae), 3 Mar. 2022, J. Boers, HPC 3835 (holotype CBS H-25200, culture ex-type CPC 42944 = CBS 149678).
Additional isolates examined: Netherlands, Friesland Province, Koehool, Zeedijk, on Physcia caesia, 3 Mar. 2022, J. Boers, HPC 3835, CBS H-25201, culture CPC 42950 = CBS 149679; ibid., CBS H-25202, culture CPC 42952 = CBS 149680.
Notes: Protocreopsis is a nectria-like genus with an acremonium-like asexual morph ( Rossman et al. 1999). Protocreopsis physciae is related to P. euphorbiae (conidiophores up to 150 μm tall, conidia (3.5–)6–7(–7.5) × (3–)4 μm; Crous et al. 2021b), but distinct in that it has larger conidia, and shorter conidiophores.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Protocreopsis euphorbiae [strain CBS 146972, GenBank NR_175202.1; Identities = 505/529 (95 %), six gaps (1 %)], Acremonium psychrophilum [voucher J. Etayo 31294, GenBank OP883927.1; Identities = 498/524 (95 %), four gaps (0 %)], Pronectria loweniae [voucher P. Rodriguez-Flakus 4000, GenBank OR116443.1; Identities = 501/534 (94 %), 11 gaps (2 %)], Protocreopsis finnmarkica [strain CBS 147428, GenBank NR_189481.1; Identities = 481/508 (95 %), four gaps (0 %)], and Protocreopsis rutila [strain JCM 23088, GenBank NR_077124.1; Identities = 494/529 (93 %), 13 gaps (2 %)]. Closest hits using the LSU sequence are Protocreopsis rutila [strain MY6, GenBank KY873378.1; Identities = 769/777 (99 %), no gaps], Protocreopsis finnmarkica [strain CBS 147428, GenBank OQ055699.1; Identities = 703/712 (99 %), no gaps], and Verruciconidia persicina [strain CBS 378.70C, GenBank HQ232080.1; Identities = 771/781 (99 %), one gap (0 %)]. Closest hits using the actA sequence of CPC 42944 had highest similarity to Protocreopsis euphorbiae [strain CPC 38896, GenBank OK651123.1; Identities = 598/671 (89 %), three gaps (0 %)], Penicillifer bipapillatus [strain CBS 420.88, GenBank KM231105.1; Identities = 369/401 (92 %), no gaps], and Parasarocladium wereldwijsianum [strain NL19094011, GenBank MW890029.1; Identities = 375/409 (92 %), no gaps]. The actA sequence of CPC 42944 is identical to those of CPC 42950 (668/668 nucleotides) and CPC 42952 (666/666 nucleotides). Distant hits obtained using the tef1 (first part) sequence of CPC 42944 had highest similarity to Thyronectria virens [strain NP10, GenBank KM225696.1; Identities = 208/238 (87 %), 11 gaps (4 %)], Thyronectria orientalis [strain 8912, GenBank KX372535.1; Identities = 206/236 (87 %), four gaps (1 %)], and Calonectria arbusta [strain CERC 5324, GenBank MF442655.1; Identities = 207/238 (87 %), six gaps (2 %)]. The tef1 sequence of CPC 42944 differs with one substitution from that of CPC 42950 (510/511 nucleotides) and is identical to that of CPC 42952 (510/510 nucleotides). Closest hits using the tub2 sequence of CPC 42944 had highest similarity to Protocreopsis euphorbiae [strain CPC 38896, GenBank OK651202.1; Identities = 367/422 (87 %), 15 gaps (3 %)], Thelonectria discophora [strain G.J.S.89-71, GenBank KC153810.1; Identities = 256/306 (84 %), 11 gaps (3 %)], and Tumenectria laetidisca [strain CBS 100284, GenBank KJ022336.1; Identities = 253/306 (83 %), 12 gaps (3 %)]. The tub2 sequence of CPC 42944 is identical to those of CPC 42950 (481/481 nucleotides) and CPC 42952 (483/483 nucleotides).
Authors: P.W. Crous, J.Z. Groenewald & J. Boers
Talaromyces podocarpi Visagie & Yilmaz, sp. nov. MycoBank MB 854287. Figs 43, 44.
Fig. 43.
Multigene phylogeny of Talaromyces sect. Islandici based on ITS, BenA, CaM and RPB2 sequences (see Suppl. Table S12). Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). Each region was treated as separate partitions, also taking into consideration introns and exons, and the nucleotide substitution model GTR+I+G was applied to each ( Abadi et al. 2019). The tree was rooted to T. subinflatus. Talaromyces podocarpi is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Fig. 44.
Talaromyces podocarpi. A. Colonies (left to right) on CYA, MEA, YES, DG18 and OA. B–D. Close-up of colonies on MEA (B), OA (C) and DG18 (D). E–J. Conidiophores. K. Conidia. Scale bars: B = 500 μm; C, D = 1 mm; E–K = 10 μm.
Etymology: Latin, podocarpi, named after Podocarpus falcatus. The species was isolated from soil collected under this tree at the “Woodville Big Tree” trail.
Classification: Ascomycota, Eurotiomycetes, Eurotiales, Trichocomaceae, Talaromyces, section Islandici.
DNA barcodes: ITS = PP375126, BenA = PP356399, CaM = PP356461, RPB2 = PP356494.
Colony diam (in mm, after 7 d, at 25 °C): CYA 10–12; CYA 10 °C no growth; CYA 15 °C 4–6; CYA 20 °C 8–10; CYA 30 °C 4–7; CYA 37 °C no growth; CYAS microcolonies; MEA 11–14; DG18 6–9; YES 12–15; OA 9–12; CREA 7–9.
Colony characters (25 °C, 7 d): Colonies on CYA moderately deep, slightly sulcate; margins low, narrow, entire; mycelia yellow; texture floccose; sporulation sparse to moderately dense, conidia en masse Greyish Green (26E5–27E5); soluble pigments absent; exudates minute clear droplets; reverse Yellowish White to Greyish Yellow to Olive Brown (4A2–B4–D5). Colonies on MEA moderately deep, plane; margins low, narrow, entire; mycelia yellow; texture floccose; sporulation very sparse, conidia en masse not determined; soluble pigments absent; exudates absent; reverse Greyish Yellow to Brown (4B4–5E8). Colonies on YES moderately deep, slightly sulcate, raised at centre; margins low, narrow, entire; mycelia yellow; texture floccose; sporulation sparse to moderately dense, conidia en masse Greyish Green (26E5–27E5); soluble pigments absent; exudates minute clear droplets; reverse Yellowish White to Greyish Yellow to Olive Brown (4A2–B4–D5). Colonies on DG18 moderately deep, plane, raised at centre; margins low, narrow, entire; mycelia white to yellow, inconspicuous; texture velutinous; sporulation moderately dense, conidia en masse Greyish Green (25D5–E5); soluble pigments absent; exudates absent; reverse Pale Yellow (2A3) to Olive (2E8–3E8). Colonies on OA moderately deep, slightly sulcate; margins low, narrow, entire; mycelia yellow; texture floccose; sporulation sparse to moderately dense, conidia en masse Greyish Green (26E5–27E5); soluble pigments absent; exudates minute clear droplets. Colonies on CREA weak growth, acid production absent.
Conidiophores biverticillate, minor proportion terverticillate, subterminal and side branches sometimes produced; stipes smooth, 25–70(–100) × 2.5–3.5 μm; branches 2–3 per stipe, 10–24 × 2.5–3.5 μm; metulae (2–)3–5 per stipe, 8–13 × 2.5–3.5 μm; phialides acerose, 4–6 per metula, 8.5–12.5 × 2.5–3.5 μm (9.8 ± 0.7 × 2.95 ± 0.2); average length metula/phialide 1.03; conidia smooth to finely roughened, globose to subglobose, 3–3.5 × 3–3.5 μm (3.3 ± 0.15 × 3.2 ± 0.2), average width/length = 0.95, n = 50.
Typus: South Africa, Western Cape Province, Wilderness, Woodville Big Tree Forest Trail (−33.934155, 22.645636), from soil, Dec. 2018, coll. M.J. Wingfield, isol. N. Yilmaz [holotype PRU(M) 4578, culture ex-type CMW-IA 001480 = CMW 60633 = CBS 152015 = CN014B2].
Additional materials examined: South Africa, Western Cape Province, Wilderness, Woodville Big Tree Forest Trail (−33.934155, 22.645636), from soil, Dec. 2018, coll. M.J. Wingfield, isol. N. Yilmaz, cultures CMW-IA 001481 = CMW 60634 = CBS 152016 = CN014B4, and CMW-IA 001482 = CMW 60635 = CBS 152017 = CN014B6.
Notes: Talaromyces podocarpi is resolved in sect. Islandici as the closest relative of T. atricola, in a clade also containing T. acaricola, T. infraolivaceus, T. neorugulosus and T. rugulosus (Fig. 43, Suppl. Fig. 4). Talaromyces podocarpi differs from other species by at least 1 bp based on ITS, 9 bp based on BenA, 7 bp based on CaM and 11 bp based on RPB2. This group of species typically shows restricted growth on most media and does not grow on CYA at 37 °C ( Yilmaz et al. 2014, Chen et al. 2016, Yilmaz et al. 2016). Compared to T. atricola, the new species has similar growth rates and colony morphologies on all media, but has a more obvious yellow mycelial colour. In addition, it produces larger (2–5 vs 3–3.5 μm) and ellipsoid (vs globose to subglobose) conidia that distinguishes it from T. atricola ( Yilmaz et al. 2014).
Authors: C.M. Visagie & N. Yilmaz
ACKNOWLEDGEMENTS
The work of P.W. Crous and colleagues benefitted from funding by the European Union’s Horizon 2020 research and innovation program (RISE) under the Marie Skłodowska-Curie grant agreement No. 101008129, project acronym “Mycobiomics”, and the Dutch NWO Roadmap grant agreement No. 2020/ENW/00901156, project “Netherlands Infrastructure for Ecosystem and Biodiversity Analysis – Authoritative and Rapid Identification System for Essential biodiversity information” (acronym NIEBA-ARISE). B. Slippers, K. Fitza and J. Allison would like to thank the Tree Protection Co-operative Programme and Natural Resources Canada for funding to support this study. C.M. Visagie would like to acknowledge the funding received from the National Research Foundation of South Africa, the Maize Trust, and the Future Leaders - African Independent Research fellowship program (FLAIR, FLR\R1\201831). The FLAIR fellowship program is a partnership between the African Academy of Sciences and the Royal Society funded by the UK Government’s Global Challenges Research Fund. Konstanze Bensch (MycoBank) is also thanked for providing guidance on some nomenclatural issues.
Conflict of interest:
The authors declare that there is no conflict of interest.
Supplementary Material: FigShare doi: 10.25403/UPresearchdata.26176783.
Single gene phylogenies of Penicillium sect. Fasciculata ser. Camembertiorum based on BenA, CaM, RPB1, RPB2, Cct8 and Tsr1. Datasets were aligned using MAFFT v. 7.520 (Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). The the most appropriate nucleotide substitution model based on the Akaike information criterion was applied to each region and its exons and introns using PartitionFinder v. 2 ( Lanfear et al. 2017). The trees were rooted to P. expansum. Penicillium dabashanicum strains are shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Single gene phylogenies of Penicillium subg. Penicillium based on ITS, BenA, CaM and RPB2. Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). The nucleotide substitution model GTR+I+G was applied to each region and its exons and introns ( Abadi et al. 2019). The trees were rooted to P. glabrum. Penicillium pascuigraminis is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Single gene phylogenies of Penicillium sect. Canescentia ser. Atroveneta based on BenA, CaM and RPB2. Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). The nucleotide substitution model GTR+I+G was applied to each region and its exons and introns ( Abadi et al. 2019). The trees were rooted to P. canescens. Penicillium viridipigmentum is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Single gene phylogenies of Talaromyces sect. Islandici based on ITS, BenA, CaM and RPB2. Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). The nucleotide substitution model GTR+I+G was applied to each region and its exons and introns ( Abadi et al. 2019). The trees were rooted to T. subinflatus. Talaromyces podocarpi is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
List of species, vouchers and GenBank accession numbers of sequences used in this study.
Strains used for phylogenetic comparisons of Anthracocystis.
GenBank accession numbers of additional isolates included in phylogenetic analyses of the genus Bisifusarium.
Strains used for phylogenetic comparisons of Devriesia and related genera.
Strains used for phylogenetic comparisons of Ericboehmia thailandica and related species.
GenBank accession numbers of additional isolates included in phylogenetic analyses of the Fusarium redolens species complex.
GenBank accession numbers of additional isolates included in phylogenetic analyses of the genus Macroconia.
Strains used for phylogenetic comparisons of Penicillium cederbergense and related species.
Strains used for phylogenetic comparisons of Penicillium dabashanicum and related species.
Strains used for phylogenetic comparisons of Penicillium pascuigraminis and related species.
Strains used for phylogenetic comparisons of Penicillium viridipigmentum and related species.
Strains used for phylogenetic comparisons of Talaromyces podocarpi and related species.
REFERENCES
- Abadi S, Azouri D, Pupko T, Mayrose I. (2019). Model selection may not be a mandatory step for phylogeny reconstruction. Nature Communications 10: 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime MC, McTaggart AR. (2021). A higher-rank classification for rust fungi, with notes on genera. Fungal Systematics and Evolution 7: 21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli A, Fry C, Smith R.et al. (2020). State of the Worlds Plants and Fungi 2020. Royal Botanical Gardens, Kew, London, UK. [Google Scholar]
- Barrera-Enríquez VP, Salazar-Yepes M. (2019). Nuevos registros de pucciniales de Colombia, incluyendo Uredendo anthurii sp. nov. y Uromyces colombiana sp. nov. Boletín Científico Centro de Museos Museo de Historia Natura 23: 95–105. [Google Scholar]
- Blackwell M. (2011). The Fungi: 1, 2, 3 … 5.1 million species? American Journal of Botany 98: 426–438. [DOI] [PubMed] [Google Scholar]
- Brefeld O. (1912). Die Brandpilze V. Untersuchungen aus dem Gesammtgebiete der Mykologie 15: 1–151. [Google Scholar]
- Chen AJ, Sun BD, Houbraken J.et al. (2016). New Talaromyces species from indoor environments in China. Studies in Mycology 84: 119–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Boers J, Holdom D.et al. (2022). Fungal Planet description sheets: 1383–1435. Persoonia 48: 261–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. (2011). Why everlastings don’t last. Persoonia 26: 70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Lombard L, Sandoval-Denis M.et al. (2021a). Fusarium: more than a node or a foot-shaped basal cell. Studies in Mycology 98: 100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Osieck ER, Jurjević Ž, et al. (2021b). Fungal Planet description sheets: 1284–1382. Persoonia 47: 178–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Osieck ER, Shivas RG.et al. (2023). Fungal Planet description sheets: 1478–1549. Persoonia 50: 158–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Rong IH, Wood A.et al. (2006a). How many species of fungi are there at the tip of Africa? Studies in Mycology 55: 13–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A.et al. (2019). New and Interesting Fungi. 2. Fungal Systematics and Evolution 3: 57–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wood AR.et al. (2020a). The Genera of Fungi - G5: Arthrinium, Ceratosphaeria, Dimerosporiopsis, Hormodochis, Lecanostictopsis, Lembosina, Neomelanconium, Phragmotrichum, Pseudomelanconium, Rutola, and Trullula. Fungal Systematics and Evolution 5: 77–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ. (2006b). Eucalyptus microfungi known from culture. 1. Cladoriella and Fulvoflamma genera nova, with notes on some other poorly known taxa. Studies in Mycology 55: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Chooi YH.et al. (2020b). Fungal Planet description sheets: 1042–1111. Persoonia 44: 301–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins GB, Hiratsuka Y. (2003). Illustrated genera of rust fungi. 3rd edn. APS Press, USA. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayarathne MC, Jones EBG, Maharachchikumbura SSN.et al. (2020). Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere 11: 1–188. [Google Scholar]
- Diekmann H. (1970). Metabolic products of microorganisms. 81. Occurrence and structures of coprogen B and dimerum acid. Archiv für Mikrobiologie 73: 65–76. [PubMed] [Google Scholar]
- Frisvad JC, Samson RA. (2004). Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Studies in Mycology 49: 1–174. [Google Scholar]
- Gardiennet A, Lechat C, Fournier J. (2019). Ericboehmia, a new genus segregated from Ostreichnion in the Hysteriaceae, with the new species E. saulensis. Ascomycete.org 11: 24. [Google Scholar]
- Gerlach W, Nirenberg HI. (1982). The genus Fusarium – A pictorial atlas. Mitteilungen der Biologischen Bundesanstalt für Land- und Forstwirtschaft 209: 1–406. [Google Scholar]
- Germishuizen G, Meyer N. (eds) (2003). Plants of southern Africa: an annotated checklist. Strelitzia 14. National Botanical Institute, Pretoria, South Africa. [Google Scholar]
- Gräfenhan T, Schroers H-J, Nirenberg HI.et al. (2011). An overview of the taxonomy, phylogeny and typification of some nectriaceous fungi classified in Cosmospora, Acremonium, Fusarium, Stilbella and Volutella . Studies in Mycology 68: 79–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Hawksworth DL. (1991). The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycological Research 95: 641–655. [Google Scholar]
- Hawksworth DL, Lucking R. (2017). Fungal Diversity Revisited: 2.2 to 3.8 million species. Microbiology Spectrum 5: FUNK-0052-2016. [DOI] [PubMed] [Google Scholar]
- Hennen JF, Figueiredo MB, de Carvalho AA.et al. (2005). Catalogue of the species of plant rust fungi (Uredinales) of Brazil. Instituto de Pesquisas, Jardim Botânico do Rio de Janeiro, Brazil. [Google Scholar]
- Houbraken J, Kocsube S, Visagie CM.et al. (2020). Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): an overview of families, genera, subgenera, sections, series and species. Studies in Mycology 95: 5–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Samson RA. (2011). Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology 70: 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Index Fungorum Partnership (2024). Species Fungorum. Royal Botanic Gardens, Kew, UK. http://www.speciesfungorum.org/Names/Names.asp. [Google Scholar]
- Jayasiri SC, Hyde KD, Jones EBG.et al. (2018). Taxonomic novelties of hysteriform Dothideomycetes. Mycosphere 9: 803–837. [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF.et al. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. (1967). Methuen Handbook of Colour. 2nd edn. Methuen & Co Ltd, London, England. [Google Scholar]
- Lanfear R, Frandsen PB, Wright AM.et al. (2017). PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. [DOI] [PubMed] [Google Scholar]
- Leslie JF, Summerell BA. (2006). The Fusarium laboratory manual . Blackwell Publishing, Iowa, USA. [Google Scholar]
- Linder DH. (1929). A monograph of the helicosporous fungi imperfecti. Annals of the Missouri Botanical Garden 16: 1–389. [Google Scholar]
- Locey KJ, Lennon JT. (2016). Scaling laws predict global microbial diversity. Proceedings of the National Academy of Sciences USA 113: 5970–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking R, Aime MC, Robbertse B.et al. (2021). Fungal taxonomy and sequence-based nomenclature. Nature Microbiology 6: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison DR, Swofford DL, Maddison WP. (1997). NEXUS: An extensible file format for systematics information. Systematic Biology 46: 590–621. [DOI] [PubMed] [Google Scholar]
- Mains EB. (1934). Angiopsora, a new genus of rusts on grasses. Mycologia 26: 122–132. [Google Scholar]
- Matsushima T. (1971). Microfungi of the Solomon Islands and Papua-New Guinea. Nippon Printing Publication Co., Kobe, Japan. [Google Scholar]
- McTaggart AR, Shivas RG, Geering AD.et al. (2012). Taxonomic revision of Ustilago, Sporisorium and Macalpinomyces. Persoonia 29: 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff WW. (1960). The Siricid wood wasps of California (Hymenoptera: Symphyta ). University of California Press, USA. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010. New Orleans, Louisiana: 1–8. [Google Scholar]
- Minh BQ, Schmidt HA, Chernomor O.et al. (2020). IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37: 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RT. (1954). Three new species of helicosporae. Mycologia 47: 19–44. [Google Scholar]
- Mucina L, Rutherford MC. (eds) (2006). The vegetation of South Africa, Lesotho, and Swaziland. Strelitzia 19. South African Biodiversity Institute, Pretoria, South Africa. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG.et al. (2000). Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Niskanen T, Lücking R, Dahlberg A.et al. (2023). Pushing the frontiers of biodiversity research: Unveiling the global diversity, distribution, and conservation of fungi. Annual Review of Environment and Resources 48: 149–176. [Google Scholar]
- Nylander JAA. (2004). MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Nilsson RH, Anslan S, Bahram M.et al. (2019). Mycobiome diversity: high-throughput sequencing and identification of fungi. Nature Reviews Microbiology 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Nilsson RH, Wurzbacher C, Bahram M.et al. (2016). Top 50 most wanted fungi. MycoKeys 12: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg HI. (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt Für Land- und Forstwirtschaft (Berlin – Dahlem ) 169: 1–117. [Google Scholar]
- O’Donnell K, Cigelnik E, Nirenberg HI. (1998). Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90: 465–493. [Google Scholar]
- Park JH, Oh J, Song JS.et al. (2019). Bisifusarium delphinoides, an emerging opportunistic pathogen in a burn patient with diabetes mellitus. Mycobiology 47: 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piątek M, Lutz M, Yorou NS. (2015). A molecular phylogenetic framework for Anthracocystis (Ustilaginales), including five new combinations (inter alia for the asexual Pseudozyma flocculosa), and description of Anthracocystis grodzinskae sp. nov. Mycological Progress 14: 88. [Google Scholar]
- Pitt JI. (1980). The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London, UK. [Google Scholar]
- Posada D, Crandall KA. (1998). MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ.et al. (2014). Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia 33: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW, Institute CM, Society BM. (1970). A Mycological Colour Chart. Commonwealth Mycological Institute, UK. [Google Scholar]
- Réblová M, Seifert KA, Fournier J.et al. (2012). Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov. and its dactylaria-like anamorph (Sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia 104: 1299–1314. [DOI] [PubMed] [Google Scholar]
- Rogerson CT, Samuels GJ. (1993). Polyporicolous species of Hypomyces. Mycologia 85: 231–272. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P.et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT.et al. (1999). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Rouget M, Reyers B, Jonas Z.et al. (2004). National Spatial Biodiversity Assessment 2004, Technical Report Volume 1: Terrestrial Component. South African National Biodiversity Institute: Pretoria, South Africa. [Google Scholar]
- Samson RA, Houbraken J, Thrane U.et al. (2019). Food and indoor fungi second edition. 2nd edn. Westerdijk Laboratory Manual Series 2. Utrecht, the Netherlands. [Google Scholar]
- Samuels GJ, Rossman AY, Lowen R.et al. (1991). A synopsis of Nectria subgen . Dialonectria. Mycological Papers 164: 1–48. [Google Scholar]
- SANBI South African National Biodiversity Institute (2007). National Biodiversity Monitoring & Reporting Framework. SANBI: Silverton, South Africa. [Google Scholar]
- Savary O, Coton E, Maillard M-B.et al. (2023). Functional diversity of Bisifusarium domesticum and the newly described Nectriaceae cheese-associated species. Food Research International 168: 112691. [DOI] [PubMed] [Google Scholar]
- Seifert KA, Nickerson NL, Corlett M.et al. (2004). Devriesia, a new hyphomycete genus to accommodate heat-resistant, cladosporium-like fungi. Canadian Journal of Botany 82: 914–926. [Google Scholar]
- Shi L, Yang H, Hyde KD.et al. (2021): Freshwater Sordariomycetes: new species and new records in Pleurotheciaceae, Pleurotheciales. Phytotaxa 518: 143–166. [Google Scholar]
- Shroers H-J, O’Donnell K, Lamprecht SC.et al. (2009). Taxonomy and phylogeny of the Fusarium dimerum species group. Mycologia 101: 44–70. [DOI] [PubMed] [Google Scholar]
- Slippers B, Coutinho TA, Wingfield BD, Wingfield MJ. (2003). A review of the genus Amylostereum and its association with woodwasps. South African Journal of Science 99: 70–74. [Google Scholar]
- Slippers B, Wingfield MJ, Wingfield BD, Coutinho TA. (2000). Relationships among Amylostereum species associated with siricid woodwasps inferred from mitochondrial ribosomal DNA sequences. Mycologia 92: 955–963. [Google Scholar]
- Smith G. (1957). Some new and interesting species of micro-fungi. Transactions of the British Mycological Society 40: 481. [Google Scholar]
- Smith G. (1961). Some new and interesting species of micro-fungi. II. Transactions of the British Mycological Society 44: 42–50. [Google Scholar]
- Smith H, Wingfield MJ, Crous PW.et al. (1996). Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany 62: 86–88. [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turland N, Wiersema J, Barrie F.et al. (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Glashütten: Koeltz Botanical Books. [Google Scholar]
- Visagie CM, Frisvad JC, Houbraken J.et al. (2021). A re-evaluation of Penicillium section Canescentia, including the description of five new species. Persoonia 46: 163–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Renaud JB, Burgess KM.et al. (2016). Fifteen new species of Penicillium. Persoonia 36: 247–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MM, Shenoy BD, Li W, Cai L. (2017). Molecular phylogeny of Neodevriesia, with two new species and several new combinations. Mycologia 109: 965–974. [DOI] [PubMed] [Google Scholar]
- Wang QM, Begerow D, Groenewald M.et al. (2015). Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Studies in Mycology 81: 55–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-C, Zhang Z-K, Zhuang W-Y. (2023). Species diversity of Penicillium in Southwest China with discovery of forty-three new species. Journal of Fungi 9: 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber HW. (1916). Fusaria Autographice Delineata. Collectio Specierum ex Herbariis Variis Selectarum et ab Auctore Lectarum Cultarumque Synonymis et Excludendis Additis quas Determinavit, in Sectiones Digessit, Comparavit cum Hypocreaceis Analogis Praemissis ad Methodi Naturalis Normas et Culturae Purae Experientiam H.W. Wollenweber 1: i, 1–11, 12a, 12b, 13–509. Germany, Berlin. [effectively published card index with lithographed text and figures]. [Google Scholar]
- Yilmaz N, Sandoval-Denis M, Lombard L.et al. (2021). Redefining species limits in the Fusarium fujikuroi species complex. Persoonia 46: 129–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz N, Visagie CM, Frisvad JC.et al. (2016). Taxonomic re-evaluation of species in Talaromyces section Islandici, using a polyphasic approach. Persoonia 36: 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz N, Visagie CM, Houbraken J.et al. (2014). Polyphasic taxonomy of the genus Talaromyces. Studies in Mycology 78: 175–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare R, Gams W. (2016). More white verticillium-like anamorphs with erect conidiophores. Mycological Progress 15: 993–1030 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Single gene phylogenies of Penicillium sect. Fasciculata ser. Camembertiorum based on BenA, CaM, RPB1, RPB2, Cct8 and Tsr1. Datasets were aligned using MAFFT v. 7.520 (Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). The the most appropriate nucleotide substitution model based on the Akaike information criterion was applied to each region and its exons and introns using PartitionFinder v. 2 ( Lanfear et al. 2017). The trees were rooted to P. expansum. Penicillium dabashanicum strains are shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Single gene phylogenies of Penicillium subg. Penicillium based on ITS, BenA, CaM and RPB2. Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). The nucleotide substitution model GTR+I+G was applied to each region and its exons and introns ( Abadi et al. 2019). The trees were rooted to P. glabrum. Penicillium pascuigraminis is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Single gene phylogenies of Penicillium sect. Canescentia ser. Atroveneta based on BenA, CaM and RPB2. Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). The nucleotide substitution model GTR+I+G was applied to each region and its exons and introns ( Abadi et al. 2019). The trees were rooted to P. canescens. Penicillium viridipigmentum is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
Single gene phylogenies of Talaromyces sect. Islandici based on ITS, BenA, CaM and RPB2. Datasets were aligned using MAFFT v. 7.520 ( Katoh & Standley 2013) and a Maximum Likelihood tree was calculated in IQ-TREE v. 2.2.2.6 ( Minh et al. 2020). The nucleotide substitution model GTR+I+G was applied to each region and its exons and introns ( Abadi et al. 2019). The trees were rooted to T. subinflatus. Talaromyces podocarpi is shown in coloured bold text. Branch support in nodes higher than 80 % bootstrap are indicated above branches (T = ex-type).
List of species, vouchers and GenBank accession numbers of sequences used in this study.
Strains used for phylogenetic comparisons of Anthracocystis.
GenBank accession numbers of additional isolates included in phylogenetic analyses of the genus Bisifusarium.
Strains used for phylogenetic comparisons of Devriesia and related genera.
Strains used for phylogenetic comparisons of Ericboehmia thailandica and related species.
GenBank accession numbers of additional isolates included in phylogenetic analyses of the Fusarium redolens species complex.
GenBank accession numbers of additional isolates included in phylogenetic analyses of the genus Macroconia.
Strains used for phylogenetic comparisons of Penicillium cederbergense and related species.
Strains used for phylogenetic comparisons of Penicillium dabashanicum and related species.
Strains used for phylogenetic comparisons of Penicillium pascuigraminis and related species.
Strains used for phylogenetic comparisons of Penicillium viridipigmentum and related species.
Strains used for phylogenetic comparisons of Talaromyces podocarpi and related species.