Abstract
Cerebral oxygen metabolism is altered in relapsing-remitting multiple sclerosis (RRMS), possibly a result of disease related cerebral atrophy with subsequent decreased oxygen demand. However, MS inflammation can also inhibit brain metabolism. Therefore, we measured cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2) using MRI phase contrast mapping and susceptibility-based oximetry in 44 patients with early RRMS and 36 healthy controls. Cerebral atrophy and white matter lesion load were assessed from high-resolution structural MRI. Expanded Disability Status Scale (EDSS) scores were collected from medical records. The CMRO2 was significantly lower in patients (−15%, p = 0.002) and decreased significantly with age in patients relative to the controls (−1.35 µmol/100 g/min/year, p = 0.036). The lower CMRO2 in RRMS was primarily driven by a higher venous oxygen saturation in the sagittal sinus (p = 0.007) and not a reduction in CBF (p = 0.69). There was no difference in cerebral atrophy between the groups, and no correlation between CMRO2 and MS lesion volume or EDSS score. Therefore, the progressive CMRO2 decline observed before the occurrence of significant cerebral atrophy and despite adequate CBF supports emerging evidence of dysfunctional cellular respiration as a potential pathogenic mechanism and therapeutic target in RRMS.
Keywords: Aging, cerebral metabolism, magnetic resonance imaging, multiple sclerosis, oxygen extraction fraction (OEF)
Introduction
Multiple sclerosis (MS) is a leading cause of non-traumatic neurological disability in young adults, and it is associated with severe health, financial and social costs.1 –3 Despite major recent developments in the number of available treatments, a substantial number of patients experience sub-clinical disease progression and ongoing cerebral atrophy, even with successful disease-modifying treatment.4,5 The main focus of multiple sclerosis disease-modifying treatment is immune modulation, but a new view of the pathophysiology may be needed because these treatments do not fully halt the disease, which may lead to novel treatment strategies.6,7 Cerebral metabolism may be one target because glucose and oxygen metabolism are affected, and the brains of multiple sclerosis patients may suffer from virtual energy failure.6,8–10 Therefore, treatment strategies targeting this perceived energy failure may improve this subtle ongoing subclinical disease progression, despite successful disease-modifying treatment.11 –13
Previous research showed that MS patients had a reduced cerebral metabolic rate of oxygen consumption (CMRO2), especially in the grey matter,14 –16 and reduced cerebral oxygen extraction.16 –18 These studies found evidence suggesting that impaired oxygen metabolism is a global phenomenon of the multiple sclerosis brain.
The underlying pathophysiology of the reduced oxygen consumption is not fully understood. However, possible explanations are decreased mitochondrial oxygen utilisation or impaired oxygen transportation through the vasculature into the brain tissue. Damage to mitochondria has been demonstrated in multiple sclerosis, but primarily progressive disease forms have been studied.19,20 Reduced cerebral glucose consumption is also described in multiple sclerosis. 10 The reduction is diffuse and not correlated to lesion load, cognitive dysfunction, or walking speed impairment.21 –26 Genes involved in the astrocyte-neuron lactate shuttle and the glutamate–glutamine cycle are downregulated in the normal-appearing grey matter of multiple sclerosis patients, which further indicate abnormal energy metabolism. 27 Bioenergetics are disrupted in MS lesions. Specifically, new lesions exhibit hypermetabolic glucose consumption, and old lesions are hypermetabolic at the rim or hypometabolic. 28 Astrocytes also show increased expression of glucose transporters in lesions, and a reduced number of axonal glucose transporters is observed in the inactive centre of chronic active lesions. 29
However, the timing and causality of these metabolic alterations are not clear, e.g., to what extent is the reduced metabolic demand a result of an atrophic brain or whether the metabolic dysfunction itself exacerbates neuronal damage. The present study addressed these questions by measuring CMRO2 and brain atrophy in a group of RRMS patients over a large age range. We examined whether patients with relapsing-remitting multiple sclerosis had a progressive impairment of CMRO2 and to what extent this impairment may be explained by brain atrophy and EDSS score.
Materials and methods
Participants
We recruited healthy controls via an online advertisement, and relapsing-remitting multiple sclerosis patients were recruited from the Multiple Sclerosis Clinic, Rigshospitalet. Forty-four patients with relapsing-remitting multiple sclerosis, aged 18–52 years (mean 35.34), and 36 healthy controls, aged 18–65 years (mean 34.3), were enrolled. The Ethics Committee of Copenhagen County approved this study, which was performed according to the standards of The National Committee on Health Research Ethics, protocol number H-1–2014–132. All experiments were performed in accordance with the Declaration of Helsinki of 1975, and all subjects gave written informed consent.
MRI
Scanner
We acquired all MRI data on a 3T magnetic resonance unit (Achieva dStream; Philips, Best, the Netherlands) using a 32-element phased-array head coil.
Cerebral blood flow and oxygen consumption
Global cerebral blood flow (CBF) was measured using phase-contrast mapping (PCM) MRI. The blood velocity in the feeding cerebral arteries (carotids and basilar arteries) was measured using PCM, which allows estimation of the total blood flow to the brain.30,31 Blood velocity weighted phase-contrast maps (example shown in Figure 1(c)) were acquired using a turbo field echo sequence (1 slice, FOV, 240 × 240 mm2; voxel size, 0.75 × 0.75 × 8 mm3 TR, 12.4 ms; TE, 7.4 ms; flip angle, 10°; velocity encoding, 100 cm/s; without cardiac gating). Two measurements were performed, one measurement with the imaging plane perpendicular to the carotid arteries and a second measurement placed perpendicular to the basilar arteries (Figure 1(a) and (b)). Blood flow was calculated by drawing regions of interest (ROIs) covering each of the feeding cerebral arteries. From these ROIs, the mean blood velocity and cross-sectional area of the arteries were found, and the flow was calculated as the product of the mean velocity and area and summed together from all feeding vessels. The total flow to the brain was normalised to brain weight to correct for different brain sizes and obtain CBF in ml/100 g/min. The brain weight was estimated from the structural images, assuming a brain density of 1.05 g/ml.
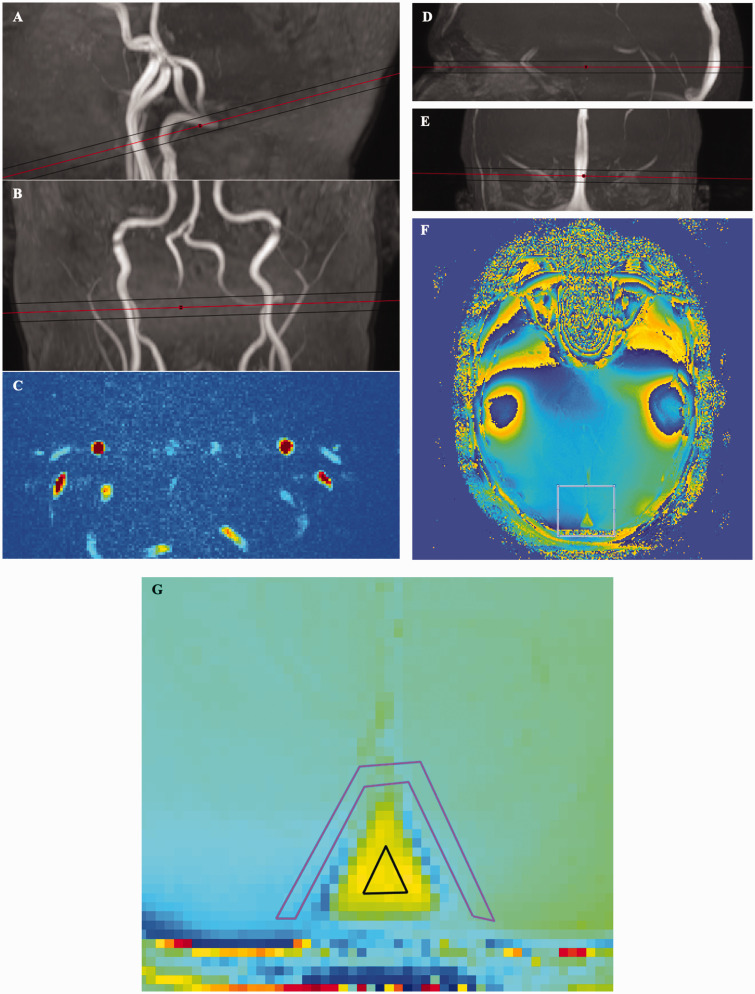
Figure 1.
The cerebral metabolic rate of oxygen consumption was determined via measurement of cerebral blood flow and venous oxygen saturation. (A and B) Angiography of internal carotid arteries showing the plane of the phase-contrast mapping slice. (C) Blood velocity map. (D and E) Venography of the superior sagittal sinus showing the plane of the susceptibility slice. (F and G) Susceptibility slice and Magnified view of the susceptibility slice showing the region of interest in the superior sagittal sinus and in the surrounding tissue.
The global cerebral metabolic rate of oxygen (CMRO2) was determined by Fick’s principle according to the following equation (1):
| (1) |
where CMRO2 is the cerebral oxygen consumption in µmol/100 g/min, [Hgb] is the haemoglobin concentration of the blood in mmol/l, CBF is the cerebral blood flow in ml/100 g/min, SaO2 is the arterial oxygen saturation, and SvO2 is the venous oxygen saturation. Haemoglobin was set to 9 mmol/l for all subjects, and arterial oxygen saturation was set to 98% for all subjects, since previous studies have shown that these do not differ significantly between MS and healthy controls. 32 We measured SvO2 in the superior sagittal sinus using a susceptibility-based oximetry (SBO) MRI technique. 33 This technique utilises venous blood, which has a different magnetic susceptibility than brain tissue, and this difference is related to oxygen saturation. The susceptibility-weighted maps were measured using a dual-echo gradient-echo sequence (1 slice, FOV, 220 × 190 mm2; voxel size, 0.5 × 0.5 × 8 mm3; TE 1, 10.89 ms; TE 2, 24.16 ms; flip angle, 30°; 5 repeated measures, total duration 1 min 30 s; SENSE-factor, 2). The difference in susceptibility between venous blood and brain tissue was found by manually drawing two ROIs, one ROI covering the superior sagittal sinus and one ROI covering the immediately surrounding tissue. The ROIs were drawn blinded to group (patient or healthy control) (Figure 1). A more in-depth discussion of the methods and data post-processing have been published previously. 34
Brain volumes, N-acetyl aspartate, and lesions
We acquired high-resolution 3D T1-weighted anatomical images with a turbo field 3D echo sequence (FOV, 256 × 256 × 179.9 mm3; voxel size, 0.7 × 0.7 × 0.7 mm3; TR, 11.3 ms; TE, 5.2 ms; flip angle, 8°). From these images, the FreeSurfer image analysis suite (FreeSurfer 7.1.0 Harvard, USA35,36) was used to estimate the CSF-free total brain volume, the brain parenchymal fraction (BPF), and segmentation of the brain images into grey and white matter. The brain parenchymal fraction was used as a measure of cerebral atrophy and was calculated as the percentage total brain volume of the estimated total intracranial volume.37,38 Grey and white matter fractions were used as measures for grey and white matter atrophy and calculated as percentage grey or white matter volumes relative to the estimated total intracranial volume.
The total lesion volume of the multiple sclerosis patients was calculated from manually delineated lesions on a T2 2D FLAIR sequence (FOV, 230 ×134.4 × 183 mm3; voxel size, 0.65 × 0.99 × 3.5 mm3 (interpolated to 0.45 × 0.45 × 3.5 mm3); TR, 11000 ms; TE, 125 ms; flip angle, 90°). HJS, who has 30+ years of experience in manual MS lesion delineation, performed the manual delineation of lesions.
We measured the concentration of lactate and the neuronal marker N-acetyl aspartate (NAA) in all patients and a subset of healthy controls (N = 24) using magnetic resonance spectroscopy in the occipital cortex using a water-suppressed point-resolved spectroscopy pulse sequence (TR, 3000 or 5000 ms; TE, 36 ms; voxel size, 30 × 35 × 30 mm3). The water concentration acquired in the spectrum was used to quantify lactate and NAA concentrations. The water concentration in the voxel was estimated from the content of grey matter, white matter, and CSF within the voxel from the segmentation of the high-resolution anatomical images.
Statistics
Statistics were computed using R (version 4.0.3). Differences in means between patients and controls of the acquired parameters were assessed using Student’s t test. When the variances were not equal according to the F test, Welch’s unequal variances t test was used. For categorical outcomes, we used Pearson's chi-squared test with Yates' continuity correction to test for significance of differences between groups.
To test for correlations between age and the acquired brain parameters, linear regression models were used. To test whether patients and controls demonstrated different effects of ageing, general linear models with an interaction term between age and disease status (multiple sclerosis yes/no) were used. The interaction term describes whether the presence of multiple sclerosis disease alters the age-related effect on the acquired parameters.
Lesion volumes were converted to a logarithmic scale to achieve a normal distribution of the data before entering the statistical modelling.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Table 1 summarises the clinical characteristics of the participants. The acquired physiology parameters and differences between patients and controls are summarised in Tables 2 and 3. Table 3 lists interaction terms for disease state in models including all subjects, whereas Figures 2 to 4 show linear correlations of parameters for healthy controls or multiple sclerosis patients, i.e. within a fixed disease state.
Table 1.
Clinical characteristics of the participants.
| Characteristic | Controls(N = 36) | Patients(N = 44) | p value |
|---|---|---|---|
| Age (mean, sd [years]) | 34.36, 14.94 | 35.69, 9.07 | 0.64 |
| Sex [% women] | 61.1% | 66.7% | 0.78 |
| EDSS (mean, range) | NA | 1.93, 0–5 | NA |
| Lesion volume (median, IQR [ml]) | NA | 7.69, 3.37–15.25 | NA |
| Disease modifying treatment | NA | 22 untreated8 teriflunomide8 dimethyl fumarate3 Interferon β1a2 fingolimod1 methotrexate | NA |
p value for Age: Welch Two Sample t-test. p value for sex: Pearson’s Chi-squared test with Yates’ continuity correction.
Table 2.
Comparisons of means in multiple sclerosis patients and healthy controls.
| Controls | Patients | Difference | 95% CI | p value | ||
|---|---|---|---|---|---|---|
| CMRO2, µmol/100 g/min | 143.18 | 121.25 | 21.94 | 7.82 | 36.05 | 0.003†,** |
| SvO2, % | 65.69 | 69.58 | −3.89 | −7.06 | −0.71 | 0.018†,* |
| CBF, ml/100 g/min | 53.17 | 52.58 | 0.59 | −3.44 | 4.61 | 0.77† |
| Brain parenchymal fraction, % | 75.66 | 74.74 | 0.92 | −0.13 | 1.96 | 0.08† |
| Grey matter fraction, % | 42.58 | 41.99 | 0.59 | −0.43 | 1.61 | 0.25†† |
| White matter fraction, % | 31.75 | 31.24 | 0.51 | −0.18 | 1.20 | 0.15† |
| NAA, mmol/l | 9.29 | 9.52 | −0.23 | −0.85 | 0.39 | 0.46†† |
*p < 0.05, **p < 0.01, †Student’s t-test, ††Welch test.
Table 3.
Summaries of disease effects of multiple sclerosis patients from general linear models relative to healthy controls.
| Model | Additional age effect in patients | 95% CI of additional effect in patients | p value | ||
|---|---|---|---|---|---|
| Age-patient interactions | |||||
| CMRO2, µmol/100 g/min/year | −1.35 | −2.587 | −0.113 | 0.036* | |
| SvO2, %/year | 0.389 | 0.115 | 0.663 | 0.007** | |
| CBF, ml/100 g/min/year | 0.213 | −0.147 | 0.573 | 0.25 | |
| BPF, %/year | 0.037 | −0.038 | 0.112 | 0.34 | |
| NAA, mmol/l/year | 0.016 | −0.018 | 0.051 | 0.36 | |
|
|
Additional CMRO2 effect in patients |
95% CI of additional effect in patients |
p value |
||
| CMRO2-patient interaction | |||||
| BPF, %/µmol/100 g/min | 0.041 | 0.008 | 0.075 | 0.018* | |
|
|
Additional NAA effect in patients |
95% CI of additional effect in patients |
p value |
||
| NAA-patient interaction | |||||
| CMRO2, µmol/100 g/min/mmol/l | −28.18 | −54.41 | −1.94 | 0.04* | |
*p < 0.05, **p < 0.01.
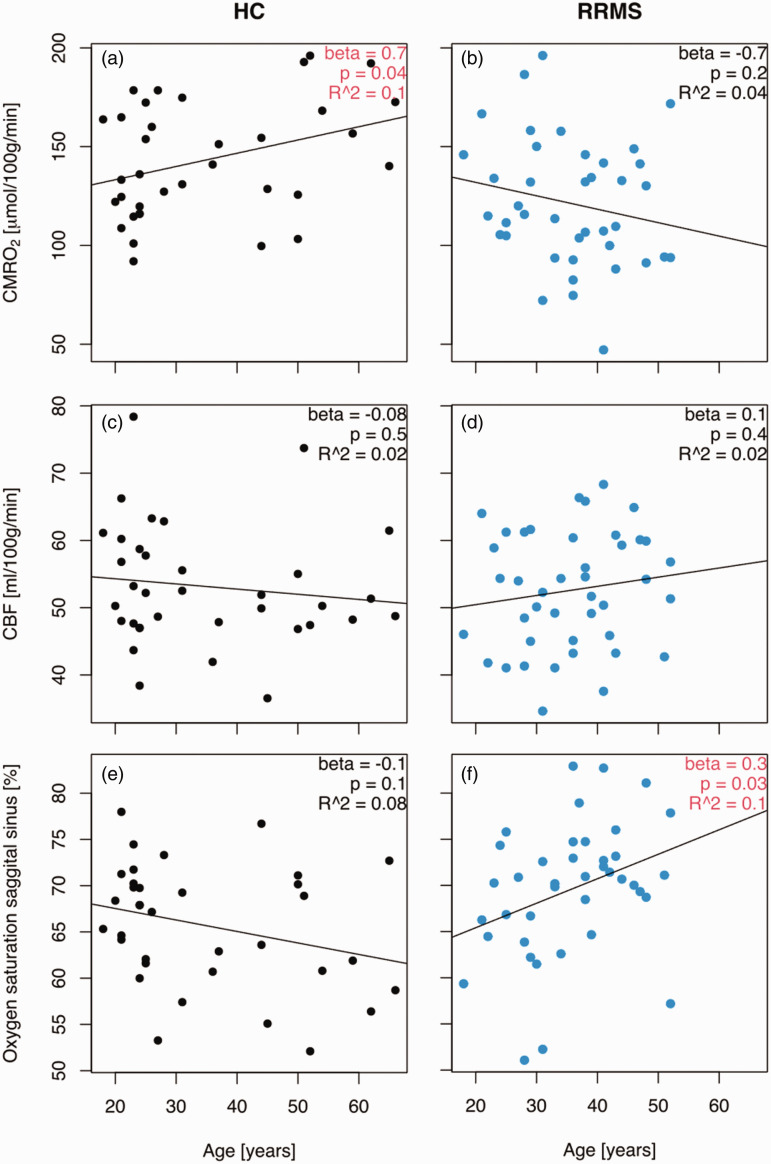
Figure 2.
Cerebral oxygen consumption declines with age in relapsing-remitting multiple sclerosis but not healthy controls. (a) The cerebral metabolic rate of oxygen consumption increased with age in healthy controls (b) and showed a trend of decline with age in relapsing-remitting multiple sclerosis patients. (c) The change in oxygen consumption was not explained by changes in cerebral blood flow because this consumption was constant across ages in healthy controls and (d) multiple sclerosis patients. (e) There was a weak trend of decline in oxygen saturation in the sagittal sinus in healthy controls and (f) and there was a significant increase in oxygen saturation in the sagittal sinus with age in MS patients. Interactions between age and disease status for the abovementioned parameters are listed in Table 3. CBF: cerebral blood flow; CMRO2: cerebral metabolic rate of oxygen consumption; HC: healthy controls; RRMS: relapsing-remitting multiple sclerosis patients. The beta coefficients represent a linear regression with a fixed disease state (HC or RRMS).
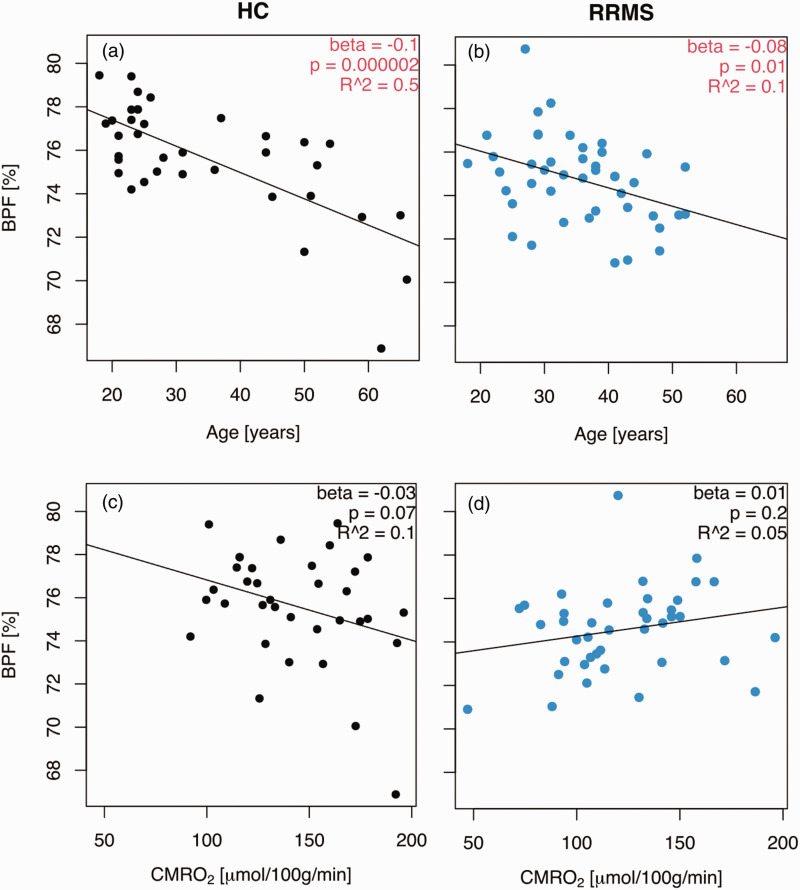
Figure 3.
Patients did not show a greater age-related atrophy than controls. (a) Brain parenchymal fraction decreased with age in healthy controls and (b) multiple sclerosis patients. (c) The brain parenchymal fraction did not correlate with the cerebral metabolic rate of oxygen consumption in healthy controls and (d) but there was a trend towards a lower brain parenchymal fraction with an increasing cerebral metabolic rate of oxygen consumption in relapsing-remitting patients. Interactions between age and disease status and between the cerebral metabolic rate of oxygen consumption and disease status for the abovementioned parameters are listed in Table 3. BPF: brain parenchymal fraction; CMRO2: cerebral metabolic rate of oxygen consumption; HC: healthy controls; RRMS: relapsing-remitting multiple sclerosis patients. The beta coefficients represent a linear regression with a fixed disease state (HC or RRMS).
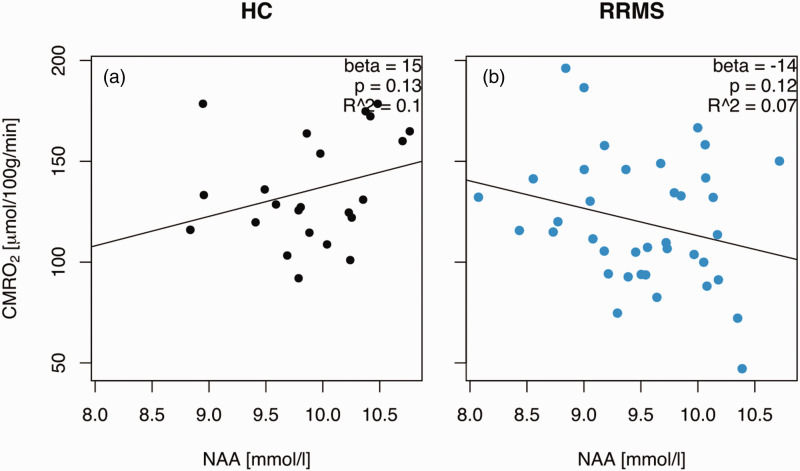
Figure 4.
CMRO2 differentially correlated with the neuronal marker N-acetyl aspartate (NAA). (a) There was a weak trend towards a decrease in the cerebral metabolic rate of oxygen consumption with increasing concentrations of NAA and (b) but there was an opposite trend in multiple sclerosis patients with an increase in the cerebral metabolic rate of oxygen consumption in MS patients. CMRO2: cerebral metabolic rate of oxygen consumption; HC: healthy controls; NAA: N-acetyl aspartate; RRMS: relapsing-remitting multiple sclerosis patients. The beta coefficients represent a linear regression with a fixed disease state (HC or RRMS).
Attrition of data
Two measurements of CBF in the multiple sclerosis patient group and one measurement from a healthy control were excluded because the acquired image was of insufficient quality due to head movement during the scan. Therefore, 42 measurements in the multiple sclerosis patient group and 35 measurements in the healthy control group were included in the final analyses.
Measurements of oxygen saturation in the sagittal sinus and blood flow in the basilar and carotid arteries were successfully performed in 40 multiple sclerosis patients (mean age 35.69 years, SD 9.07) and 35 healthy controls (mean age 34.36 years, SD 14.94) and included in the final analyses. Four measurements from multiple sclerosis patients and one measurement from a healthy control were excluded due to excessive inhomogeneity of the acquired phase MRI images.
Thirty-nine multiple sclerosis patients and 25 healthy controls completed magnetic resonance spectroscopy measurements of NAA and lactate. Five of these measurements from patients were excluded due to poor magnetic resonance spectra. All NAA and lactate measurements from healthy controls were included in the final analyses.
The EDSS score was available in the medical records of 43 patients.
Cerebral physiology
The mean CMRO2 was significantly (p = 0.003) higher in healthy controls compared to patients (controls: 143.2 µmol/100 g/min (SD: 29.2); patients: 121.2 µmol/100 g/min (SD: 32.1)) (Table 2). CMRO2 increased significantly with age in the control group (mean effect: +0.7 µmol/100 g/min per year, 95% CI: 0.02 to 1.32 µmol/100 g/min/year, p = 0.04), but the patient group showed a weak trend towards a decrease with age (mean effect: −0.68 µmol/100 g/min per year, 95% CI: −1.81 to 0.45 µmol/100 g/min/year, p = 0.2). The two trajectories were significantly different, as modelled by the interaction (mean effect 1.35 µmol/100 g/min per year, 95% CI: 0.11 to 2.59 µmol/100 g/min/year, p = 0.036) (Figure 2(a) and (b)). Therefore, multiple sclerosis patients demonstrated a reduction in CMRO2 with advancing age compared to healthy controls.
There was no significant difference (p = 0.78) in CBF between patients and controls (controls: 53.17 ml/100 g/min (SD: 8.95; patients: 52.58 ml/100 g/min (SD: 8.66)). We did not observe any correlations between CBF and age in either group (p = 0.5 for controls and p = 0.4 for patients) (Figure 2(c) and (d)) or any difference in the age-related trajectories between the two groups (p = 0.25) (Table 3). Therefore, no change in CBF was observed in the multiple sclerosis patients.
The oxygen saturation of the blood leaving the brain in the sagittal sinus was significantly higher in patients compared to controls (patients: 69.58% (SD: 7.23); controls: 65.70% (SD: 6.57), p = 0.02). Increasing age was associated with increasing oxygen saturation in the sagittal sinus in the patient group (mean effect: 0.3% per year, 95% CI: 0.02 to 0.51%/year, p = 0.03), and this effect was significantly larger compared to the healthy controls (0.39% per year, 95% CI: 0.12 to 0.66%/year, p = 0.007). Therefore, cerebral oxygen extraction was decreased in multiple sclerosis patients with advancing age compared to healthy controls (Figure 2(e) and (f)).
Brain volumes
The mean brain parenchymal fraction (BPF) was not significantly (p = 0.08) different between the groups (controls: 75.66% (SD: 2.57); patients: 74.74% (SD: 1.98)). The BPF decreased with age (p < 0.01) and at a similar (p = 0.34) rate in patients and controls, at 0.1% per year for controls and 0.08% per year for patients (Table 3 and Figure 3(a) and (b)). There were no significant differences in grey matter fraction between patients and controls (p = 0.25) (controls: 42.58% (SD: 2.61); patients: 41.99% (SD: 1.77)), and the grey matter fraction decreased with age in both groups (controls: 0.15% per year, 95% CI: 0.18 to 0.12, p < 0.001; patients: 0.09, 95% CI: 0.14 to 0.03, p = 0.002). The white matter fraction was similar (p = 0.15) in patients and controls (controls: 31.75% (SD: 1.55); patients: 31.24% (SD: 1.53)) and did not decrease with age (controls: p = 0.9; patients: p = 0.9).
BPF tended to decrease with increasing CMRO2 in the control group (−0.03% per µmol/100 g/min, 95% CI: −0.06 to 0.002, p = 0.07), but there was a weak change in the opposite direction in patients, although non-significant (0.01% per µmol/100 g/min, 95% CI: −0.006 to 0.032, p = 0.2), such that BPF and CMRO2 were significantly differently correlated for patients relative to controls (p = 0.02) (Table 3) (Figure 3(c) and (e)).
Therefore, the relationship between brain atrophy and age was comparable between healthy controls and multiple sclerosis patients. Higher CMRO2 in MS patients indicated less brain atrophy relative to controls.
Energy metabolism markers N-acetyl aspartate (NAA) and lactate
There was no difference (p = 0.46) in the concentration of NAA in patients and controls (controls: 9.89 mmol/l (SD: 0.58); patients: 9.52 mmol/l (SD: 0.59)).
The CMRO2 in patients changed differently as a function of NAA relative to healthy controls (−28.2 µmol/100 g/min per mmol/l NAA relative to controls, 95% CI: −54.4 to −1.9, p = 0.039), see Figure 4. For healthy controls, there was a weak trend towards a CMRO2 increase as a function of the concentration of NAA (mean effect: 14.6 µmol/100 g/min per mmol/l, 95% CI: −4.9 to 34.1, p = 0.14), but the trend was in the opposite direction in patients (−13.6 µmol/100 g/min per mmol/l, 95% CI: −30.9 to 3.8, p = 0.12).
Therefore, the neuronal marker NAA was oppositely related to cerebral oxygen consumption in multiple sclerosis patients and controls despite no difference in the concentration of NAA relative to age.
Clinical and imaging markers of disease severity
Patients were minimally to moderately affected by their disease, with EDSS scores between 0 and 5 (mean 1.9) and a median T2 lesion volume of 7.69 ml (interquartile range 3.37 to 15.25 ml). The CMRO2 was not correlated with T2 lesion volume (p = 0.97) or EDSS score (p = 0.23) (Supplementary 1). The multiple sclerosis patients did not show evidence of vascular lesions or enlarged Virchow-Robin spaces.
Discussion
We observed an age-dependent decrease in CMRO2 in relapsing-remitting multiple sclerosis patients compared to healthy controls. The lower CMRO2 was explained by a reduced cerebral oxygen extraction, which was evident by higher venous oxygen saturation in the sagittal sinus of the patients compared to healthy controls. The reduced CMRO2 was not explained by atrophy because the patients did not demonstrate a lower BPF compared to the control group. However, age-related atrophy seemed associated with a higher CMRO2 in controls but not patients, which amplified the difference in CMRO2 relative to brain parenchymal fraction between the groups.
We observed a decrease in CMRO2 with increasing concentrations of NAA in multiple sclerosis patients compared to healthy controls. This result was not expected. NAA is synthesized in the mitochondria of neuronal axons39,40 and transferred to oligodendrocytes. 41 It is involved in neuronal energy metabolism, and NAA has been shown to decrease with oxygen consumption and ATP production when the electron transport chain was inhibited in rat neuronal cultures.42,43 NAA is decreased in the context of demyelination in MS, but it recovers when plaques resolve and patients receive disease-modifying treatment.9,42 Therefore, our observation may be a spurious and random finding or a result of mitochondrial dysfunction in multiple sclerosis.
Generally, our findings support the evidence of impaired oxygen metabolism as a potential pathogenic mechanism in relapsing-remitting multiple sclerosis, and the impairment seemed to become accentuated as a function of age in multiple sclerosis patients.
The decreasing CMRO2 we observed in multiple sclerosis patients may indicate dysfunctional mitochondrial oxygen utilisation. Alternatively, it may be related to alterations in oxygen transportation across the capillary wall. Oxygen consumption in the healthy ageing brain is relatively stable or increases slightly with age when corrected for the age related decrease in brain size, which is incorporated in the term CMRO2.44 –47 The observed increase in CMRO2 in healthy individuals with age is likely a result of inefficient mitochondrial energy production due to proton and electron leaks in the electron transport chain.48,49
Previous rodent and human studies support the hypothesis of reduced oxygen utilisation by the mitochondria in multiple sclerosis. Specifically, a reduced number and morphology of the mitochondria, oxidative damage to mitochondrial DNA in plaques, and altered transport capabilities of the mitochondria have been observed.19,50,51 Reduced activity of mitochondrial electron transport chain complexes has been observed in rodent models of multiple sclerosis.11,12,52,53 This reduction in the activity of electron transport chain complexes was also confirmed in human post-mortem brain tissue52,54–57 and patients with clinically isolated syndrome, a precursor for multiple sclerosis. Genes controlling mitochondria and electron transport chain complexes are downregulated in the cerebrospinal fluid from multiple sclerosis patients. 58 Elevated biomarkers of mitophagy have been found in the cerebrospinal fluid and serum from MS patients with active disease, which suggests an increased turnover of mitochondria.59,60 Metabolomic analysis of urine from multiple sclerosis patients have shown alterations in metabolites related to energy and fatty acid metabolism and mitochondrial activity. 61 This mitochondrial dysfunction may exacerbate the axonal loss observed in multiple sclerosis patients. 62 Mouse neurons decrease the transcription of genes related to the electron transport chain when exposed to inflammatory stimuli. Neurodegeneration was exacerbated in a mouse model of multiple sclerosis when the gene Ppargc1a, which increases the number of mitochondria, complex IV activity, and maximum respiratory capacity, was deleted and ameliorated when the same gene was overexpressed. 12 Inflammation in multiple sclerosis leads to elevated levels of nitrogen oxide (NO),63 –65 which inhibits the utilisation of oxygen in complex IV of the electron transport chain. 66
Reduced oxygen diffusion over the capillary is another potential explanation for a lower CMRO2. The blood-brain barrier (BBB) has increased solute permeability in relapsing-remitting multiple sclerosis. 67 Pericytes govern the integrity of the BBB and regulate perfusion at the capillary level. Dysfunction of pericytes disturbs the perfusion pattern and results in higher capillary transit time heterogeneity, which impedes oxygen transport into tissue. Local inflammation along vessels also increase diffusion distances. 68 Future studies could addressed this issue using dynamic contrast-enhanced MRI. 69 It is unlikely that the decreased CMRO2 is due to age-dependent arteriosclerotic vascular pathology because the lesions of RRMS patients were not compatible with typical large or small vessel brain disease. We did not identify enlarged Virchow-Robin spaces, lacunar infarctions, or white matter hyperintensity lesions in the basal ganglia. 70
Differences in BPF between patients and controls did not explain the decreased CMRO2. The brains of multiple sclerosis patients swell due to inflammation.71,72 Because CMRO2 by definition is normalised to brain volume, swelling with increased volume but no additional metabolically active cells would present as decreased CMRO2. However, the oxygen saturation in the sagittal sinus, which is not normalised to BPF, followed the same trend as the CMRO2, and swelling is not a likely explanation for the decreased CMRO2 in the present study. Notably, NAA, which has been used as a marker of neurons but is produced in the mitochondria, showed an opposite correlation to CMRO2 in RRMS patients compared to healthy controls. This finding supports mitochondrial defects and not a lack of mitochondria as the underlying explanation for our findings, which may be a compensatory increase in NAA.
The measured levels of CMRO2, CBF and oxygen saturation in the sagittal sinus are consistent with previous MRI studies in healthy controls and relapsing-remitting multiple sclerosis patients, but previous studies did not report any potential relationships between these factors and age.14 –16,18,31,73,74 Specifically, a recent study finds similar decreases in global CMRO2 and increases in global venous oxygen saturation for grey matter in relapsing-remitting multiple sclerosis. 75 Their modelling estimates a higher partial pressure of oxygen at the mitochondria in the patients, which suggest reduced oxygen demand as an explanation for the lower CMRO2. In contrast to our study, this study reports lower grey matter CBF in patients compared to age/sex matched controls, and negative correlations between lesion volume and both cortical CMRO2, and lesion volume and cortical CBF. The populations studied were of similar age, sex distribution, fraction of patients receiving disease modifying treatment and EDSS score. First, this discrepancy between findings could be a result of a different methodological approach, since Chandler et al. utilized arterial spin labeling and only reported regional CBF for grey matter, whereas we utilized PCM for the assessment of global CBF, the former method yielding overall lower CBF values. 76 Furthermore, Chandler, et al. excluded patients who had a recent change in medication, relapse or steroid treatment. We did not impose similar restrictions and therefore our results may to a higher degree reflect current inflammatory activity, which has previously been shown to coincide with higher CBF values. 77 The Chandler et al. study did not examine whether there was a relationship between age and CMRO2, but our study covered a greater age range and included twice the number of participants, which may have made this discovery possible. 75 Previously reported correlations between EDSS and total lesion volume and the measurements of CMRO2 and oxygen saturation in the sagittal sinus are ambiguous.15 –18,74,75
If a pathological gradual decrease in CMRO2 is present in multiple sclerosis, targeting cerebral metabolism may be a new treatment strategy. A recent in vitro study showed that supporting the mitochondrial function of neurons increased remyelination, 11 but currently no treatments exist for relapsing-remitting multiple sclerosis that addresses metabolic dysfunction. 78 Further studies addressing serial CMRO2 measurements in the context of multiple sclerosis disease-modifying treatment, relapses and atrophy development over time would be highly beneficial.
Strengths and limitations
One main strength of the study is that we examined a cohort consisting of only relapsing-remitting multiple sclerosis subjects, which contrasts with previous work that, except for one study, 18 focused on clinically definite multiple sclerosis14,15 or investigated a mixed population of relapsing-remitting and progressive multiple sclerosis patients.16,17 A wide age range was included, which increased the external validity of our findings.
There are also some limitations to the present study. For the determination of the CMRO2, we would like to address two assumptions. The arterial oxygen saturation was assumed to be 98% in all participants, whether healthy or sick. If multiple sclerosis patients had a lower arterial oxygen saturation, this factor would be a competing explanation for the decreased cerebral oxygen consumption. However, we are not aware of any evidence of MS patients having a lower arterial oxygen saturation, and previous studies on oxygen extraction and venous oxygen saturation in multiple sclerosis assumed that patients had the same arterial oxygen saturation as healthy controls.16,17 Future studies can measure arterial oxygen saturation using pulse-based oximetry if they are interested in ameliorating this potential difference. We also assumed that patients and controls had a haemoglobin concentration of 9 mmol/l, consistent with previous work finding no difference in haemoglobin between healthy controls and early MS. 79 Women of reproductive age have a lower haemoglobin concentration than men due to monthly blood loss in relation to their menstrual periods, and older patients are more likely to have a lower haemoglobin concentration due to undiscovered disease, such as bleeding in the gastrointestinal canal. These differences would have implications for the interpretation of the results. However, we consider these risks to be balanced between patients and controls because the age and sex of patients and controls were similar.
The present study measured the oxygen saturation in the sagittal sinus and interpreted it as the venous oxygen saturation of the entire brain. However, the sagittal sinus only drains approximately 50% of the cerebrum and the falx cerebri and pericranium. We normalised the total CMRO2 measurement to the total brain volume. This normalisation introduces a bias if the inferior cerebrum or the cerebellum are differently affected by multiple sclerosis than the cerebrum. Normalising the CMRO2 to total brain volume is generally used,33,80 and previous studies used a similar approach16,17 or found no significant regional differences between the cerebellum and cerebrum.
Interpretation of the spectroscopic measurements of NAA and lactate are primarily limited by two conditions. The first condition is age. The healthy controls with magnetic resonance spectroscopic data were an average of 8.6 years younger than the patients. Our data showed no significant effects of age on the concentration of NAA or lactate, but the concentrations of both metabolites changes with age. 46 Therefore, we only made age-adjusted comparisons between patients and controls. However, any changes in multiple sclerosis from middle age and beyond would go undetected in our study. The second condition is that we only acquired magnetic resonance spectroscopy in the occipital lope, and thus we cannot draw conclusions about NAA or lactate concentrations in other parts of the brain.
Conclusion
The present study supports the evidence of mitochondrial dysfunction as a potential pathogenic mechanism in relapsing-remitting multiple sclerosis. Whether reduced cerebral oxygen consumption predicts future atrophy and may be used as an early biomarker require further study.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231224502 for Age-related decline in cerebral oxygen consumption in multiple sclerosis by Maria H Knudsen, Mark B Vestergaard, Ulrich Lindberg, Helle J Simonsen, Jette L Frederiksen, Stig P Cramer and Henrik BW Larsson in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors thank the neurologists and multiple sclerosis patients from the multiple sclerosis clinic at Rigshospitalet. The authors also thank radiographers B.S. Møller, K.E. Segers and R.H. Tavangar.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Sanofi Genzyme [Grant number GZ-2016-11629] and The Danish Multiple Sclerosis Society [Grant numbers A37989, A40212, A41682]. The funding bodies had no influence on the study design, inclusion of patients, data analysis, interpretation or writing of the final manuscript.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare the following potential conflicts of interest with respect to the research, authorship and/or publication of this article. M.H. Knudsen received funding from Sanofi Genzyme and The Danish Multiple Sclerosis Society and non-financial support from Merck. H.B.W. Larsson & S.P. Cramer received funding from Sanofi Genzyme and The Danish Multiple Sclerosis Society. Sanofi Genzyme had no influence on the study design, inclusion of patients, data analysis or interpretation. U. Lindberg, M.B. Vestergaard and H.J. Simonsen have nothing to disclose. J.L. Frederiksen received no funding to support this study. She has served on scientific advisory boards for and received funding for travel related to these activities and honoraria from Biogen Idec, Merck Serono, Sanofi-Aventis, Teva, Novartis and Almirall. She has also received speaker honoraria from Biogen Idec, Teva and Novartis. She has served as an advisor on preclinical development for Takeda. J. L Frederiksen participated in advisory board meetings with Alexion and Chiesi.
Authors’ contributions: MHK, MBV, UL, SPC and HBWL contributed to the design of the study, acquisition, analysis and interpretation of the data, and the drafting of the article. HJS contributed to the acquisition and analysis of the data and the drafting of the article. JLF contributed to the acquisition and interpretation of the data and the drafting of the article. All authors critically revised the draft and approved the version to be published.
Supplementary material: Supplemental material for this article is available online.
ORCID iDs: Maria H Knudsen https://orcid.org/0000-0003-0432-1488
Ulrich Lindberg https://orcid.org/0000-0002-0004-6354
References
- 1.Jennum P, Wanscher B, Frederiksen J, et al. The socioeconomic consequences of multiple sclerosis: a controlled national study. Eur Neuropsychopharmacol 2012; 22: 36–43. [DOI] [PubMed] [Google Scholar]
- 2.Ysrraelit C, Caceres F, Villa A, et al. ENCOMS: Argentinian survey in cost of illness and unmet needs in multiple sclerosis. Arq Neuropsiquiatr 2014; 72: 337–343. [DOI] [PubMed] [Google Scholar]
- 3.Dahham J, Rizk R, Kremer I, et al. Economic burden of multiple sclerosis in low- and Middle-Income countries: a systematic review. Pharmacoeconomics 2021; 39: 789–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sastre-Garriga J, Pareto D, Battaglini M, et al. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol 2020; 16: 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 2017; 389: 1347–1356. [DOI] [PubMed] [Google Scholar]
- 6.Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet 2018; 391: 1622–1636. [DOI] [PubMed] [Google Scholar]
- 7.Bierhansl L, Hartung H-P, Aktas O, et al. Thinking outside the box: non-canonical targets in multiple sclerosis. Nat Rev Drug Discov 2022; 21: 578–600. doi:10.1038/s41573-022-00477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol 2009; 8: 280–291. [DOI] [PubMed] [Google Scholar]
- 9.Paling D, Golay X, Wheeler-Kingshott C, et al. Energy failure in multiple sclerosis and its investigation using MR techniques. J Neurol 2011; 258: 2113–2127. [DOI] [PubMed] [Google Scholar]
- 10.Mathur D, Rodas GL, Casanova B, et al. Perturbed glucose metabolism: insights into multiple sclerosis pathogenesis. Front Neurol 2014; 5: 250–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Licht-Mayer S, Campbell GR, Canizares M, et al. Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis. Acta Neuropathol 2020; 140: 143–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenkranz SC, Shaposhnykov AA, Träger S, et al. Enhancing mitochondrial activity in neurons protects against neurodegeneration in a mouse model of multiple sclerosis. Elife 2021; 10: 1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picone P, Nuzzo D. Promising treatment for multiple sclerosis: mitochondrial transplantation. Int J Mol Sci 2022; 23: 2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks DJ, Leenders KL, Head G, et al. Studies on regional cerebral oxygen utilisation and cognitive function in multiple sclerosis. J Neurol Neurosurg Psychiatry 1984; 47: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Tanaka M, Kondo S, et al. Clinical significance of reduced cerebral metabolism in multiple sclerosis: a combined PET and MRI study. Ann Nucl Med 1998; 12: 89–94. [DOI] [PubMed] [Google Scholar]
- 16.Ge Y, Zhang Z, Lu H, et al. Characterizing brain oxygen metabolism in patients with multiple sclerosis with T2 –Relaxation-Under-Spin-Tagging MRI. J Cereb Blood Flow Metab 2012; 32: 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan AP, Govindarajan ST, Kinkel RP, et al. Quantitative oxygen extraction fraction from 7-Tesla MRI phase: reproducibility and application in multiple sclerosis. J Cereb Blood Flow Metab 2015; 35: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho J, Nguyen TD, Huang W, et al. Brain oxygen extraction fraction mapping in patients with multiple sclerosis. J Cereb Blood Flow Metab 2022; 42: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Barcelos IP, Troxell RM, Graves JS. Mitochondrial dysfunction and multiple sclerosis. Biology (Basel) 2019; 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adiele RC, Adiele CA. Metabolic defects in multiple sclerosis. Mitochondrion 2019; 44: 7–14. [DOI] [PubMed] [Google Scholar]
- 21.Blinkenberg M, Jensen CV, Holm S, et al. A longitudinal study of cerebral glucose metabolism, MRI, and disability in patients with MS. Neurology 1999; 53: 149–153. [DOI] [PubMed] [Google Scholar]
- 22.Blinkenberg M, Rune K, Jensen CV, et al. Cortical cerebral metabolism correlates with MRI lesion load and cognitive dysfunction in MS. Neurology 2000; 54: 558–564. [DOI] [PubMed] [Google Scholar]
- 23.Derache N, Marié RM, Constans JM, et al. Reduced thalamic and cerebellar rest metabolism in relapsing-remitting multiple sclerosis, a positron emission tomography study: correlations to lesion load. J Neurol Sci 2006; 245: 103–109. [DOI] [PubMed] [Google Scholar]
- 24.Roelcke U, Kappos L, Lechner-Scott J, et al. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 1997; 48: 1566–1571. [DOI] [PubMed] [Google Scholar]
- 25.Bakshi R, Miletich RS, Kinkel PR, et al. High-resolution fluorodeoxyglucose positron emission tomography shows both global and regional cerebral hypometabolism in multiple sclerosis. J Neuroimaging 1998; 8: 228–234. [DOI] [PubMed] [Google Scholar]
- 26.Kindred JH, Tuulari JJ, Bucci M, et al. Walking speed and brain glucose uptake are uncoupled in patients with multiple sclerosis. Front Hum Neurosci 2015; 9: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeis T, Allaman I, Gentner M, et al. Metabolic gene expression changes in astrocytes in multiple sclerosis cerebral cortex are indicative of immune-mediated signaling. Brain Behav Immun 2015; 48: 313–325. [DOI] [PubMed] [Google Scholar]
- 28.Schiepers C, Van Hecke P, Vandenberghe R, et al. Positron emission tomography, magnetic resonance imaging and proton NMR spectroscopy of white matter in multiple sclerosis. Mult Scler 1997; 3: 8–17. [DOI] [PubMed] [Google Scholar]
- 29.Nijland PG, Michailidou I, Witte ME, et al. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 2014; 62: 1125–1141. [DOI] [PubMed] [Google Scholar]
- 30.Bakker CJG, Hartkamp MJ, Mali WPTM. Measuring blood flow by nontriggered 2D phase-contrast MR angiography. Magn Reson Imaging 1996; 14: 609–614. [DOI] [PubMed] [Google Scholar]
- 31.Vestergaard MB, Lindberg U, Aachmann-Andersen NJ, et al. Comparison of global cerebral blood flow measured by phase-contrast mapping MRI with 15 O-H 2 O positron emission tomography. J MAGN Reson IMAGING 2017; 45: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vestergaard MB, Henriksen OM, Lindberg U, et al. No evidence for direct effects of recombinant human erythropoietin on cerebral blood flow and metabolism in healthy humans. J Appl Physiol (1985) 2018; 124: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 33.Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab 2010; 30: 1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vestergaard MB, Larsson HB. Cerebral metabolism and vascular reactivity during breath-hold and hypoxic challenge in freedivers and healthy controls. J Cereb Blood Flow Metab 39: 834–848. doi:10.1177/0271678X17737909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 36.Reuter M, Schmansky NJ, Rosas HD, et al. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012; 61: 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vågberg M, Granåsen G, Svenningsson A. Brain parenchymal fraction in healthy adults-a systematic review of the literature. PLoS One 2017; 12: e0170018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 2014; 28: 147–156. [DOI] [PubMed] [Google Scholar]
- 39.Truckenmiller ME, Namboodiri MAA, Brownstein MJ, et al. N‐acetylation of L‐aspartate in the nervous system: differential distribution of a specific enzyme. J Neurochem 1985; 45: 1658–1662. [DOI] [PubMed] [Google Scholar]
- 40.Ariyannur PS, Moffett JR, Manickam P, et al. Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: implications for specialized acetyl coenzyme a metabolism in the CNS. Brain Res 2010; 1335: 1–13. [DOI] [PubMed] [Google Scholar]
- 41.Nordengen K, Heuser C, Rinholm JE, et al. Localisation of N-acetylaspartate in oligodendrocytes/myelin. Brain Struct Funct 2015; 220: 899–917. [DOI] [PubMed] [Google Scholar]
- 42.Moffett JR, Ross B, Arun P, et al. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 2007; 81: 89–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bates TE, Strangward M, Keelan J, et al. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport 1996; 7: 1397–1400. [PubMed] [Google Scholar]
- 44.Peng SL, Dumas JA, Park DC, et al. Age-related increase of resting metabolic rate in the human brain. Neuroimage 2014; 98: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibaraki M, Shinohara Y, Nakamura K, et al. Interindividual variations of cerebral blood flow, oxygen delivery, and metabolism in relation to hemoglobin concentration measured by positron emission tomography in humans. J Cereb Blood Flow Metab 2010; 30: 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vestergaard MB, Jensen MLF, Arngrim N, et al. Higher physiological vulnerability to hypoxic exposure with advancing age in the human brain. J Cereb Blood Flow Metab 2020; 40: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aanerud J, Borghammer P, Chakravarty MM, et al. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab 2012; 32: 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001; 21: 1133–1145. [DOI] [PubMed] [Google Scholar]
- 49.Zhao RZ, Jiang S, Zhang L, et al. Mitochondrial electron transport chain, ROS generation and uncoupling (review). Int J Mol Med 2019; 44: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu F, Selak M, O'Connor J, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci 2000; 177: 95–103. [DOI] [PubMed] [Google Scholar]
- 51.Kiryu-Seo S, Ohno N, Kidd GJ, et al. Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochondrial transport. J Neurosci 2010; 30: 6658–6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahad DJ, Ziabreva I, Campbell G, et al. Mitochondrial changes within axons in multiple sclerosis. Brain 2009; 132: 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadeghian M, Mastrolia V, Rezaei Haddad A, et al. Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci Rep 2016; 6: 33249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandit A, Vadnal J, Houston S, et al. Impaired regulation of electron transport chain subunit genes by nuclear respiratory factor 2 in multiple sclerosis. J Neurol Sci 2009; 279: 14–20. [DOI] [PubMed] [Google Scholar]
- 55.Campbell GR, Ziabreva I, Reeve AK, et al. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann Neurol 2011; 69: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell GR, Mahad DJ. Clonal expansion of mitochondrial DNA deletions and the progression of multiple sclerosis. CNS Neurol Disord Drug Targets 2012; 11: 589–597. [DOI] [PubMed] [Google Scholar]
- 57.Campbell GR, Reeve AK, Ziabreva I, et al. No excess of mitochondrial DNA deletions within muscle in progressive multiple sclerosis. Mult Scler 2013; 19: 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lutz NW, Viola A, Malikova I, et al. Inflammatory multiple-sclerosis plaques generate characteristic metabolic profiles in cerebrospinal fluid. PLoS One 2007; 2: e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cossu D, Yokoyama K, Sechi LA, et al. Potential of PINK1 and PARKIN proteins as biomarkers for active multiple sclerosis: a Japanese cohort study. Front Immunol 2021; 12: 681386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patergnani S, Fossati V, Bonora M, et al. Mitochondria in multiple sclerosis: molecular mechanisms of pathogenesis. Int Rev Cell Mol Biol 2017; 328: 49–103. [DOI] [PubMed] [Google Scholar]
- 61.Gebregiworgis T, Nielsen HH, Massilamany C, et al. A urinary metabolic signature for multiple sclerosis and neuromyelitis optica. J Proteome Res 2016; 15: 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell GR, Worrall JT, Mahad DJ. The central role of mitochondria in axonal degeneration in multiple sclerosis. Mult Scler 2014; 20: 1806–1813. [DOI] [PubMed] [Google Scholar]
- 63.Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol 2002; 1: 232–241. [DOI] [PubMed] [Google Scholar]
- 64.Encinas JM, Manganas L, Enikolopov G. Nitric oxide and multiple sclerosis. Curr Neurol Neurosci Rep 2005; 5: 232–238. [DOI] [PubMed] [Google Scholar]
- 65.Broholm H, Andersen B, Wanscher B, et al. Nitric oxide synthase expression and enzymatic activity in multiple sclerosis. Acta Neurol Scand 2004; 109: 261–269. [DOI] [PubMed] [Google Scholar]
- 66.Moncada S, Bolaños JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem 2006; 97: 1676–1689. [DOI] [PubMed] [Google Scholar]
- 67.Cramer SP, Simonsen H, Frederiksen JL, et al. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. NeuroImage Clin 2014; 4: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Angleys H, Østergaard L, Jespersen SN. The effects of capillary transit time heterogeneity (CTH) on brain oxygenation. J Cereb Blood Flow Metab 2015; 35: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larsson HBW, Vestergaard MB, Lindberg U, et al. Brain capillary transit time heterogeneity in healthy volunteers measured by dynamic Contrast-Enhanced T 1-weighted perfusion MRI. J MAGN Reson IMAGING 2017; 45: 1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geraldes R, Ciccarelli O, Barkhof F, et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol 2018; 14: 199–213. [DOI] [PubMed] [Google Scholar]
- 71.Vidal-Jordana A, Sastre-Garriga J, Rovira A, et al. Treating relapsing–remitting multiple sclerosis: therapy effects on brain atrophy. 2015. [DOI] [PubMed]
- 72.Andravizou A, Dardiotis E, Artemiadis A, et al. Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Auto Immun Highlights 2019; 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madsen SS, Lindberg U, Asghar S, et al. Reproducibility of cerebral blood flow, oxygen metabolism, and lactate and N-acetyl-aspartate concentrations measured using magnetic resonance imaging and spectroscopy. Front Physiol 2023; 14: 1213352–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vestergaard MB, Frederiksen JL, Larsson HBW, et al. Cerebrovascular reactivity and neurovascular coupling in multiple sclerosis – a systematic review. Front Neurol 2022; 13: 912828–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chandler HL, Stickland RC, Patitucci E, et al. Reduced brain oxygen metabolism in patients with multiple sclerosis: evidence from dual-calibrated functional MRI. J Cereb Blood Flow Metab 2023; 43: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dolui S, Wang Z, Wang DJJ, et al. Comparison of non-invasive MRI measurements of cerebral blood flow in a large multisite cohort. J Cereb Blood Flow Metab 2016; 36: 1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bester M, Forkert ND, Stellmann JP, et al. Increased Perfusion in Normal Appearing White Matter in High Inflammatory Multiple Sclerosis Patients. PLoS One 2015; 10: e0119356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cunniffe N, Coles A. Promoting remyelination in multiple sclerosis. J Neurol 2021; 268: 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cramer SP, Modvig S, Simonsen HJ, et al. Permeability of the blood-brain barrier predicts conversion from optic neuritis to multiple sclerosis. Brain 2015; 138: 2571–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med 2009; 62: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231224502 for Age-related decline in cerebral oxygen consumption in multiple sclerosis by Maria H Knudsen, Mark B Vestergaard, Ulrich Lindberg, Helle J Simonsen, Jette L Frederiksen, Stig P Cramer and Henrik BW Larsson in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.