Abstract
Introduction
Carbazochrome sodium sulfonate (CSS) is a hemostatic agent that reduces capillary permeability and enhances capillary resistance. However, its specific effects on colorectal endoscopic submucosal dissection (ESD) outcomes remain uncertain. This study aimed to assess the risk factors for post-ESD bleeding and the effect of CSS on colorectal ESD outcomes.
Methods
First, we retrospectively analyzed the risk factors for post-ESD bleeding using data from 1,315 lesions in 1,223 patients who underwent ESD for superficial colorectal neoplasms at eight institutions. Second, patients were divided into CSS and non-CSS groups using propensity score matching, and their outcomes from colorectal ESD were analyzed.
Results
The risk factors for post-colorectal ESD bleeding were identified as age of ≥70 years, tumor located in the rectum, tumor size of ≥40 mm, and post-ESD defect unclosure in both univariate and multivariate analyses. The CSS and non-CSS groups each consisted of 423 lesions after propensity score matching. The post-colorectal ESD bleeding rate was 3.5% (15/423) and 3.3% (14/423) in the CSS and non-CSS groups, respectively, indicating no significant differences. Among patients with the high-risk factors for post-ESD bleeding, the administration of CSS also did not demonstrate a significant reduction in the post-ESD bleeding rate compared to the non-CSS group.
Conclusion
CSS administration is ineffective in preventing post-colorectal ESD bleeding in both the general population and individuals at a high risk for such bleeding. Our results indicate the necessity to reconsider the application of CSS for preventing post-colorectal ESD bleeding.
Keywords: Bleeding, Risk factor, Colon, Endoscopic submucosal dissection, Carbazochrome sodium sulfonate
Introduction
Endoscopic submucosal dissection (ESD) has achieved a higher en bloc resection rate and lower local recurrence rate for colorectal neoplasms compared to endoscopic mucosal resection [1–3]. However, colorectal ESD poses technical challenges and carries the risk of complications such as post-ESD bleeding [4]. The reported incidence of postoperative bleeding in colorectal ESD is 2.7–4.3%, which is lower than the rates observed in gastric ESD (5–10%) and duodenal ESD (5–17%) [5]. While postoperative bleeding in colorectal ESD is relatively infrequent, the need for emergent endoscopy to achieve hemostasis can still pose a burden for both patients and endoscopists. Moreover, post-colorectal ESD bleeding has the potential to progress to hemorrhagic shock, necessitating blood transfusion and surgical intervention [5, 6]. Recent reports suggest that endoscopic closure of mucosal defects after colorectal ESD may decrease the incidence of such bleeding [7–9]. However, the utilization of endoscopic closure comes with added costs and extended procedural time. When considering the incidence of postoperative bleeding in colorectal ESD, it is recommended to apply this approach to patients at a high risk for post-ESD bleeding [10]. In terms of injectable drugs, there are currently no established pharmaceutical interventions for preventing postoperative bleeding in colorectal ESD. Hence, there is a need to elucidate the impact of medications with hemostatic properties in the context of colorectal ESD.
Carbazochrome sodium sulfonate (CSS) is a hemostatic agent that works by decreasing capillary permeability and increasing capillary resistance, thereby reducing bleeding time [11, 12]. Conventionally, CSS has been used to control bleeding in the gastrointestinal tract, including bleeding associated with ESD. In surgical and endoscopic resection contexts, CSS administration has reduced intraoperative and postoperative blood loss in orthopedic surgery; however, its hemostatic effect is considered inadequate for decreasing the occurrence rate of postoperative bleeding in gastric ESD [12, 13]. Therefore, the research on the hemostatic effects of CSS is currently inconclusive, and the specific impact of CSS in colorectal ESD remains uncertain. This study was conducted to elucidate the risk factors for post-ESD bleeding and to investigate the effects of CSS on the outcomes of colorectal ESD.
Materials and Methods
Study Patients
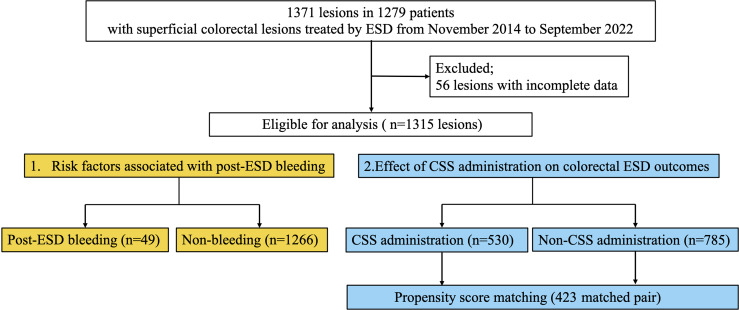
This retrospective study included 1,371 consecutive lesions of 1,279 patients with superficial colorectal neoplasms that underwent ESD at eight institutions, including Asahikawa Medical University Hospital, Asahikawa City Hospital, Asahikawa-Kosei General Hospital, Nayoro City General Hospital, Japanese Red Cross Asahikawa Hospital, Sapporo Higashi Tokushukai Hospital, Furano Kyokai Hospital, and Engaru-Kosei General Hospital, from November 2014 to September 2022. We excluded 56 lesions with incomplete data. Finally, this study enrolled 1,315 lesions in 1,223 patients with colorectal neoplasms (Fig. 1). This study underwent a centralized review by the Ethics Committee of Asahikawa Medical University and received approval from each institution. Informed consent was obtained using an opt-out method for this retrospective study.
Fig. 1.
Study flowchart.
Pre-ESD Management of Antithrombotic Agents
We consulted the prescribing doctor to determine the feasibility of discontinuing the antithrombotic agents before ESD for patients receiving antithrombotic treatment. When antithrombotic agent withdrawal was deemed appropriate, antithrombotic agent discontinuation followed the regulations of each hospital and the guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment [14, 15]. Aspirin was discontinued for 3–5 days before the ESD procedure. Thienopyridine derivatives were halted 5–7 days before the procedure. Warfarin was withdrawn for 3–4 days before the ESD procedure, and heparin replacement was withheld until the morning of the procedure. Direct oral anticoagulants and other antiplatelet agents were withdrawn on the morning of the procedure. Antithrombotic agents were continued during the perioperative period when the prescribing doctor determined that withdrawal was inappropriate.
ESD Procedures
Experts with experience in conducting over 50 ESD procedures or non-experts who were under the guidance of experts at each institution performed ESD. A single-channel lower gastrointestinal endoscope (PCF-Q260J or PCF-H290ZI; Olympus Medical Systems, Tokyo, Japan) was used with a high-frequency generator (VIO3, VIO-300D, or VIO-200D; Erbe Elektromedizin GmbH, Tübingen, Germany). The endoscopists utilized an electrosurgical tip knife device, specifically a FlushKnife (Fujifilm, Tokyo, Japan) and a DualKnife (Olympus Medical Systems, Tokyo, Japan). Hyaluronic acid solution (MucoUp; Boston Scientific Corporation, Tokyo, Japan or K smart; Olympus Medical Systems, Tokyo, Japan) or sodium alginate (Liftal K; Kaigen Pharma Co., Ltd., Osaka, Japan) was injected into the submucosal (SM) layer to lift the mucosa. The mucosal incision and SM dissection were initiated from the proximal side and continued to the distal side. Then, en bloc resection was performed. The endoscopists assessed the artificial ulcer site to confirm the absence of any bleeding from exposed blood vessels immediately after ESD. Post-ESD defect was sutured using an EZ clip (Olympus, Tokyo, Japan) and a repositionable clip (SureClip; Micro-Tech Co. Ltd., Nanjing, China).
CSS Administration and Post-ESD Management
CSS was administered at three institutions, including Asahikawa Medical University Hospital, Asahikawa-Kosei General Hospital, and Nayoro City General Hospital, and was not administered at the remaining five institutions. CSS was intravenously administered before the ESD procedure and continued until postoperative day 1 or 2, with a dosage of either 50 mg/day or 100 mg/day. Patients maintained a fasting state after ESD. Blood tests and physical examinations were conducted on postoperative day 1. An emergency endoscopy was performed in cases of hematochezia occurring on postoperative days (Fig. 2). We promptly conducted emergency endoscopic hemostasis when active bleeding was confirmed from the post-ESD defects. Our study defined post-ESD bleeding as a lower gastrointestinal hemorrhage that requires endoscopic hemostasis or is accompanied by a decrease in hemoglobin (Hb) levels of ≥2 mg/dL, as outlined in the guideline of the Japan Gastroenterological Endoscopy Society [16]. The timing of antithrombotic agent resumption was determined by the endoscopists after endoscopic hemostasis. In cases with no abnormal blood tests or physical examinations, antithrombotic agents were resumed on the day after the ESD procedure and oral intake was reintroduced on postoperative day 2. Patients were discharged from our hospital on postoperative days 4–6.
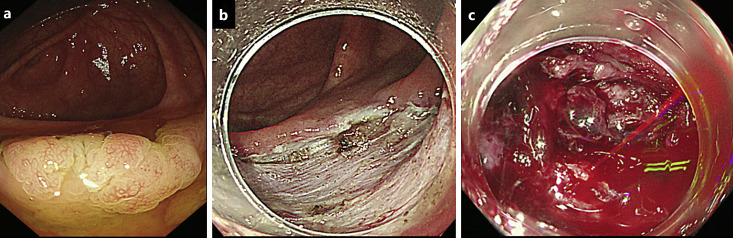
Fig. 2.
A case showing the post-ESD bleeding. A 28-mm LST was detected in the cecum, which was pathologically diagnosed as an SSL with dysplasia (a). The post-ESD defect with the majority of vessels ablated was observed in the cecum (b). The post-ESD bleeding was observed at the post-ESD defect site (c). LST, laterally spreading tumor; SSL, sessile serrated lesion.
Propensity Score Matching
Propensity score matching was conducted to address potential confounding biases between the CSS and non-CSS groups. The propensity score model was estimated using logistic regression, adjusting for variables such as age, sex, comorbidities (atrial fibrillation, ischemic heart disease, cerebrovascular disease, and chronic kidney disease on hemodialysis), medication intake (aspirin, thienopyridine derivatives, cilostazol, warfarin, direct oral anticoagulants, and other antithrombotic agents), antithrombotic agent intake (single, double, and triplet therapy), continued anticoagulant use during ESD, heparin replacement, expertise, preoperative Hb levels, lesion characteristics (location, morphology, pathology, SM invasion, fibrosis, and tumor size), and closure status of post-ESD defects. The two groups were matched in a 1:1 ratio based on their propensity scores.
Statistical Analyses
The R Project for Statistical Computing version 4.0.5 software program was used for all statistical analyses. Student’s t test was used to compare continuous variables and Fisher’s exact probability test for nominal scale data. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to evaluate the strength of the influence of each variable. The multivariate analysis included selected variables with p values of <0.05 in univariate analysis. p values of <0.05 were considered statistically significant.
Results
Risk Factors of Post-ESD Bleeding
Table 1 shows the clinicopathological features of the post-ESD bleeding and non-bleeding groups for all lesions. The overall rate of post-ESD bleeding was 3.7% (49/1,315). A significantly higher prevalence of age of ≥70 years, cerebrovascular disease, intake of thienopyridine derivatives, tumor located at the rectum, tumor size of ≥40 mm, and unclosure of post-ESD defects was observed in the post-ESD bleeding group compared to the non-bleeding group. The post-ESD bleeding caused significantly reduced Hb levels measured after hematochezia (from 13.9 g/dL to 12.0 g/dL), whereas the non-bleeding group exhibited a relatively minor decrease (from 13.6 g/dL to 13.1 g/dL). Other factors (gender, continued anticoagulant use, CSS administration, endoscopists, macroscopic type, histology, depth of invasion, presence of fibrosis, resection time, en bloc resection, intraoperative perforation, and delayed perforation) did not differ between the post-ESD bleeding and non-bleeding groups. Additionally, in the non-bleeding group, 2.6% (33 out of 1,266 lesions) were treated with a combination of CSS and tranexamic acid, showing no significant difference compared to the post-ESD bleeding group. Regarding the onset of post-ESD bleeding, 42.9% (21 out of 49 lesions) exhibited early-phase bleeding within 24 h of the procedure, while 57.1% (28 out of 49 lesions) presented with late-phase bleeding occurring after 24 h. The mean standard deviation onset of post-ESD bleeding was 2.1 (2.8) days. Among the 49 patients who experienced post-ESD bleeding, 5 patients (10.2%) required blood transfusions, and 8 patients (16.3%) exhibited a systolic blood pressure drop below 90 mm Hg, indicative of hypovolemic shock. The post-ESD bleeding cases were managed with endoscopic hemostasis, and there were no cases requiring interventional radiology, surgery, intensive care, or resulting in death.
Table 1.
Clinicopathological features of the post-ESD bleeding and non-bleeding groups
| Post-ESD bleeding | Non-bleeding | p value | |
|---|---|---|---|
| Lesions/patients, n | 49/49 | 1,266/1,174 | |
| Age, mean (SD), years | 72.9 (9.0) | 69.5 (10.4) | 0.02 |
| Age of ≥70 years, n (%) | 36 (73.5) | 683 (53.9) | <0.01 |
| Gender, n (%) | 0.37 | ||
| Male | 34 (69.4) | 730 (62.2) | |
| Female | 15 (30.6) | 444 (37.8) | |
| Comorbidity, n (%) | |||
| Atrial fibrillation | 4 (8.2) | 57 (4.5) | 0.28 |
| Ischemic heart disease | 2 (4.1) | 63 (5.0) | >0.99 |
| Cerebrovascular disease | 9 (18.4) | 93 (7.3) | 0.01 |
| Chronic kidney disease in HD | 1 (2.0) | 3 (0.2) | 0.14 |
| Antithrombotic therapy, n (%) | |||
| Aspirin | 4 (8.2) | 90 (7.1) | 0.78 |
| Thienopyridine derivatives | 6 (12.2) | 47 (3.7) | 0.01 |
| Cilostazol | 2 (4.1) | 40 (3.2) | 0.67 |
| Warfarin | 1 (2.0) | 21 (1.7) | 0.57 |
| DOAC | 5 (10.2) | 65 (5.1) | 0.18 |
| Others | 2 (4.1) | 56 (4.4) | >0.99 |
| Antithrombotic agent use, n (%) | |||
| Single | 7 (14.3) | 181 (14.3) | >0.99 |
| Doublet | 5 (10.2) | 53 (4.2) | 0.06 |
| Triplet | 1 (2.0) | 9 (0.7) | 0.32 |
| Heparin replacement, n (%) | 3 (6.1) | 20 (1.6) | 0.051 |
| Continuation of anticoagulant use, n (%) | |||
| Aspirin | 0 (0) | 9 (0.7) | >0.99 |
| Thienopyridine derivatives | 0 (0) | 1 (0.1) | >0.99 |
| Cilostazol | 0 (0) | 6 (0.5) | >0.99 |
| Warfarin | 0 (0) | 2 (0.2) | >0.99 |
| DOAC | 0 (0) | 1 (0.1) | >0.99 |
| CSS administration, n (%) | 18 (36.7) | 512 (40.4) | 0.66 |
| Tranexamic acid administration, n (%) | 0 (0) | 33 (2.6) | 0.63 |
| Expert, n (%) | 41 (83.7) | 1,028 (81.2) | 0.85 |
| Hb, mean (SD), g/dL | |||
| Preoperative Hb | 13.9 (2.1) | 13.6 (1.7) | 0.28 |
| Postoperative Hb on POD1 | 13.1 (2.2) | 13.1 (1.7) | 0.99 |
| Postoperative Hb (lowest data) | 12.0 (2.3) | 13.1 (1.7) | <0.01 |
| Tumor location, n (%) | <0.01 | ||
| Right-side colon | 16 (32.7) | 670 (52.9) | |
| Left-side colon | 10 (20.4) | 271 (21.4) | |
| Rectum | 23 (46.9) | 325 (25.7) | |
| Macroscopic type, n (%) | 0.60 | ||
| LST-G | 22 (44.9) | 563 (44.5) | |
| LST-NG | 19 (38.8) | 423 (33.4) | |
| Protruded | 8 (16.3) | 280 (22.1) | |
| Histology, n (%) | |||
| Tubular adenoma | 16 (32.7) | 483 (38.2) | 0.22 |
| Adenocarcinoma, n (%) | 29 (59.2) | 666 (52.6) | |
| SSL | 3 (6.1) | 113 (8.9) | |
| SSL with dysplasia | 1 (2.0) | 4 (0.3) | |
| Depth of invasion, n (%) | 0.83 | ||
| SM | 5 (10.2) | 153 (12.1) | |
| Fibrosis, n (%) | 0 (0) | 89 (7.0) | 0.07 |
| Tumor size, mean (SD), mm | 31.1 (17.7) | 25.6 (14.0) | 0.01 |
| Tumor size of ≥40 mm, n (%) | 13 (26.5) | 161 (12.7) | <0.01 |
| Resection time, mean (SD), minutes | 84.0 (89.5) | 70.3 (57.6) | 0.11 |
| En bloc resection, n (%) | 43 (87.8) | 1,189 (93.9) | 0.12 |
| Intraoperative perforation, n (%) | 0 (0) | 47 (3.7) | 0.42 |
| Delayed perforation, n (%) | 1 (2.0) | 20 (1.6) | 0.55 |
| Endoscopic suture, n (%) | <0.01 | ||
| Suture of post-ESD defects | 2 (4.1) | 245 (19.4) | |
| Unclosure of post-ESD defects | 47 (95.9) | 1,021 (80.6) | |
HD, hemodialysis; DOAC, direct oral anticoagulant; ESD, endoscopic submucosal dissection; P-CAB, potassium-competitive acid blocker; UL, ulceration; SD, standard deviation; CSS, carbazochrome sodium sulfonate; LST, laterally spreading tumor; SSL, sessile serrated lesion; Hb, hemoglobin.
Table 2 summarizes the univariate and multivariate analyses for factors associated with post-ESD bleeding. The univariate analysis revealed significant risk factors and characteristics as follows: age of ≥70 years (OR: 2.36, 95% CI: 1.21–4.90; p < 0.01), cerebrovascular disease (OR: 2.83, 95% CI: 1.17–6.17; p = 0.01), intake of thienopyridine derivatives (OR: 3.61, 95% CI: 1.20–9.12; p = 0.02), tumor located at the rectum (OR: 2.56, 95% CI: 1.37–4.91; p < 0.01), tumor size of ≥40 mm (OR: 2.48, 95% CI: 1.18–4.91; p < 0.01), and unclosure of post-ESD defects (OR: 5.64, 95% CI: 1.36–23.4; p < 0.01). The multivariate analysis revealed age of ≥70 years (OR: 2.12, 95% CI: 1.10–4.09; p = 0.03), tumor located at the rectum (OR: 2.14, 95% CI: 1.18–3.87; p = 0.01), tumor size of ≥40 mm (OR: 2.29, 95% CI: 1.16–4.51; p = 0.02), and unclosure of post-ESD defects (OR: 5.63, 95% CI: 1.46–48.21; p < 0.01) as independent risk factors for post-ESD bleeding.
Table 2.
Univariate and multivariate analyses for factors of post-ESD bleeding
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age of ≥70 years | 2.36 | 1.21–4.90 | <0.01 | 2.12 | 1.10–4.09 | 0.03 |
| Cerebrovascular disease | 2.83 | 1.17–6.17 | 0.01 | 1.67 | 0.70–4.00 | 0.25 |
| Thienopyridine derivatives | 3.61 | 1.20–9.12 | 0.02 | 2.66 | 0.94–7.52 | 0.07 |
| Tumor location: rectum | 2.56 | 1.37–4.74 | <0.01 | 2.14 | 1.18–3.87 | 0.01 |
| Tumor size of ≥40 mm | 2.48 | 1.18–4.91 | <0.01 | 2.29 | 1.16–4.51 | 0.02 |
| Unclosure of post-ESD defects | 5.63 | 1.46–48.21 | <0.01 | 4.69 | 1.12–19.60 | 0.03 |
CI, confidence interval; ESD, endoscopic submucosal dissection; OR, odds ratio.
Effect of CSS Administration on Colorectal ESD Outcomes
Table 3 shows the clinicopathological features of the CSS and non-CSS groups after propensity score matching. The CSS and non-CSS groups consisted of 423 lesions each. In the CSS group, 24 (5.7%) and 399 (94.3%) lesions were treated with a CSS dosage of 50 mg/day and 100 mg/day, respectively. The CSS administration duration was 0–1 and 0–2 days postoperatively in 89 (21.0%) and 334 (79.0%) lesions, respectively. The post-ESD bleeding rate was 3.5% (15/423) and 3.3% (14/423) in the CSS and non-CSS groups. The rate of early-phase bleeding within 24 h of the procedure was 0.9% (4/423) in the CSS group and 2.1% (9/423) in the non-CSS group, which did not show the significant differences. Both the CSS and non-CSS groups exhibited similar preoperative Hb levels (13.8 g/dL and 13.7 g/dL, respectively) and comparable postoperative Hb levels on the first postoperative day (13.0 g/dL and 13.2 g/dL, respectively).
Table 3.
Clinicopathological features of the CSS and non-CSS groups after propensity score matching
| CSS group | Non-CSS group | p value | |
|---|---|---|---|
| Lesions, n (%) | 423 | 423 | |
| Age, mean (SD), years | 69.8 (9.7) | 69.6 (10.9) | 0.81 |
| Gender, n (%) | 0.83 | ||
| Male | 259 (61.2) | 263 (62.2) | |
| Female | 164 (38.8) | 160 (37.8) | |
| Comorbidity, n (%) | |||
| Atrial fibrillation | 20 (4.7) | 15 (3.5) | 0.49 |
| Ischemic heart disease | 21 (5.0) | 25 (5.9) | 0.65 |
| Cerebrovascular disease | 31 (7.3) | 35 (8.3) | 0.70 |
| Chronic kidney disease in HD, n (%) | 0 (0) | 0 (0) | – |
| Antithrombotic therapy, n (%) | |||
| Aspirin | 32 (7.6) | 33 (7.8) | >0.99 |
| Thienopyridine derivatives | 15 (3.5) | 17 (4.0) | 0.86 |
| Cilostazol | 13 (3.1) | 15 (3.5) | 0.85 |
| Warfarin | 4 (0.9) | 6 (1.4) | 0.75 |
| DOAC | 18 (3.5) | 15 (4.3) | 0.72 |
| Others | 17 (4.0) | 21 (4.7) | 0.74 |
| Antithrombotic agent use, n (%) | |||
| Single | 62 (14.7) | 59 (13.9) | 0.84 |
| Doublet | 15 (3.5) | 16 (3.8) | 0.87 |
| Triplet | 2 (0.5) | 4 (0.9) | 0.69 |
| Heparin replacement, n (%) | 7 (1.7) | 11 (2.6) | 0.48 |
| Expert, n (%) | 350 (82.7) | 365 (86.3) | 0.18 |
| CSS, n (%) | – | ||
| 50 mg | 24 (5.7) | 0 (0) | |
| 100 mg | 399 (94.3) | 0 (0) | |
| CSS duration, n (%) | – | ||
| Day 0–1 | 89 (21.0) | 0 (0) | |
| Day 0–2 | 334 (79.0) | 0 (0) | |
| Hb, mean (SD), g/dL | |||
| Preoperative Hb | 13.8 (1.7) | 13.7 (1.7) | 0.44 |
| Postoperative Hb on POD1 | 13.0 (1.7) | 13.2 (1.7) | 0.07 |
| Tumor location, n (%) | 0.87 | ||
| Right-side colon | 205 (48.5) | 210 (49.6) | |
| Left-side colon | 98 (23.2) | 100 (23.6) | |
| Rectum | 120 (28.4) | 113 (26.7) | |
| Macroscopic type, n (%) | 0.99 | ||
| LST-G | 203 (48.0) | 201 (47.5) | |
| LST-NG | 128 (30.3) | 128 (30.3) | |
| Protruded | 92 (21.7) | 94 (22.2) | |
| Histology, n (%) | >0.99 | ||
| Tubular adenoma | 185 (43.7) | 199 (43.7) | |
| Adenocarcinoma | 200 (47.3) | 185 (47.0) | |
| SSL | 38 (9.0) | 39 (9.0) | |
| SSL with dysplasia | 0 (0) | 0 (0) | |
| Depth, n (%) | 0.85 | ||
| SM | 63 (14.9) | 60 (14.2) | |
| Fibrosis, n (%) | 25 (5.9) | 27 (6.4) | 0.89 |
| Tumor size, mean (SD), mm | 26.5 (15.2) | 25.9 (13.2) | 0.52 |
| Resection time, mean (SD), minutes | 71.6 (54.8) | 68.6 (57.9) | 0.43 |
| En bloc resection, n (%) | 415 (98.1) | 390 (92.2) | <0.01 |
| Post-ESD bleeding, n (%) | 15 (3.5) | 14 (3.3) | >0.99 |
| Post-ESD bleeding within 24 h, n (%) | 4 (0.9) | 9 (2.1) | 0.26 |
| Intraoperative perforation, n (%) | 12 (2.8) | 20 (4.7) | 0.21 |
| Delayed perforation, n (%) | 7 (1.7) | 9 (2.1) | 0.80 |
| Endoscopic suture, n (%) | 0.73 | ||
| Suture of post-ESD defects | 79 (18.7) | 84 (19.9) | |
| Unclosure of post-ESD defects | 344 (81.3) | 339 (80.1) | |
CSS, carbazochrome sodium sulfonate; ESD, endoscopic submucosal dissection; HD, hemodialysis; LST, laterally spreading tumor; SD, standard deviation; SM, submucosal; SSL, sessile serrated lesion; Hb, hemoglobin; DOAC, direct oral anticoagulant.
In the analysis comparing different CSS dosages (50 mg/day and 100 mg/day), no significant differences were observed in terms of the occurrence rate and onset of post-ESD bleeding, as well as postoperative Hb levels (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000539367). Furthermore, among patients with high-risk factors for post-ESD bleeding, such as an age of ≥70 years, tumor located in the rectum, tumor size of ≥40 mm, and unclosure of post-ESD defects, the administration of CSS did not show a significant reduction in the post-ESD bleeding rate compared to the non-CSS group (Table 4). Thus, CSS administration was shown to be ineffective in decreasing post-ESD bleeding.
Table 4.
Incidence of post-ESD bleeding in high-risk patients between the CSS and non-CSS groups
| Post-ESD bleeding | CSS | Non-CSS | p value |
|---|---|---|---|
| Age of ≥70 years, n (%) | 11/284 (3.9) | 25/435 (5.7) | 0.30 |
| Tumor location: rectum, n (%) | 9/155 (5.8) | 14/193 (7.3) | 0.67 |
| Tumor size of ≥40 mm, n (%) | 7/68 (10.3) | 6/106 (5.7) | 0.38 |
| Unclosure of post-ESD defects, n (%) | 17/384 (4.4) | 30/684 (4.4) | >0.99 |
ESD, endoscopic submucosal dissection; CSS, carbazochrome sodium sulfonate.
Regarding colorectal ESD outcomes, the en bloc resection rate was significantly higher in the CSS group (98.1%) compared to the non-CSS group (92.2%) (Table 3). The average resection time was 71.6 min in the CSS group and 68.6 min in the non-CSS group, showing no significant differences. The rates of intraoperative and delayed perforation did not exhibit significant differences between the CSS and non-CSS groups. As for the complication of CSS administration, no instances of thrombosis were observed in either the CSS group or the non-CSS group.
Discussion
This is the first report to reveal that CSS administration is ineffective in preventing post-colorectal ESD bleeding in both the general population and individuals at a high risk for such bleeding. Conventionally, CSS administration was employed for hemostasis during the perioperative periods of colorectal ESD. However, our results suggest the necessity to reevaluate the application of CSS for preventing post-colorectal ESD bleeding.
Previous reports indicate that combining intraoperative and 3-h postoperative CSS administration with tranexamic acid significantly decreases both intraoperative blood loss and total blood loss on postoperative day 2 in orthopedic surgery [17]. However, our study revealed that CSS administration demonstrated no significant reduction in the occurrence rate of post-ESD bleeding, despite being administered to 89 (21.0%) lesions 0–1 day postoperatively and 334 (79.0%) lesions 0–2 days postoperatively. The post-ESD defects are subjected to stimulation from peristalsis and the passage of stool unlike maintaining rest at the surgical site of orthopedic surgery. This stimulation may cause insufficient effectiveness of CSS administration in preventing post-ESD bleeding, while the endoscopic suture of post-ESD defects was proven effective in preventing such bleeding by avoiding these stimulations. In addition, CSS is designed to provide a hemostatic effect by targeting capillary vessels, but most capillary vessels are usually ablated during colorectal ESD [11]. Hence, the hemostatic effect of CSS for preventing postoperative bleeding may be weakened in colorectal ESD.
Regarding colorectal ESD outcomes, the en bloc resection rate was significantly higher in the CSS group (98.1%) than in the non-CSS group (92.2%). If CSS administration reduces intraoperative bleeding and enhances the en bloc resection rate, its impact is noteworthy. However, our retrospective study lacks measurements of intraoperative bleeding severity and hemorrhage time during the ESD procedures. Additionally, postoperative Hb levels exhibited no significant differences between the CSS and non-CSS groups. There exists inadequate evidence to establish that the hemostatic effect of CSS contributes to an enhanced en bloc resection rate in this study. Therefore, further prospective research is necessary to validate the hemostatic impact of CSS on the improvement of the en bloc resection rate.
We also identified risk factors associated with post-colorectal ESD bleeding, including being ≥70 years old, having a tumor located in the rectum, possessing a tumor size of ≥40 mm, and leaving post-ESD defects unclosed. Elderly patients encompass a wider range of potential factors associated with post-ESD bleeding compared to their younger counterparts [18]. Our findings demonstrated that elderly patients (≥70 years old) were identified as a risk factor for post-colorectal ESD bleeding. Rectal location is consistently indicated as a significant risk factor for post-colorectal ESD bleeding in many studies [5, 19–21]. The presence of abundant vasculature, intraluminal pressures, and stretching may contribute to post-colorectal ESD bleeding, particularly in cases located in the rectum [5]. A larger tumor size could lead to a larger post-ESD ulcer, increased exposure of SM vessels, and a prolonged period for mucosal regeneration and healing [22]. These factors collectively may contribute to the occurrence of post-colorectal ESD bleeding in cases of larger tumor size.
This study has several limitations. First, it was a retrospective study, which inherently carries the risk of selection bias and limited control over confounding factors. However, we attempted to mitigate these limitations by including a substantial sample size from multiple centers and using propensity score matching. Second, CSS was administered at three institutions, including the university hospital, while it was not administered at the other five institutions. Despite our utilization of propensity score matching, this institutional disparity could potentially impact the clinicopathological characteristics, particularly in relation to the rate of en bloc resection. Third, the blood collection date for measuring the preoperative Hb level was based on the outpatient visit and was not standardized. Measuring the preoperative Hb level immediately before the ESD procedure would be desirable to accurately compare the changes in postoperative Hb levels. Fourth, our study lacked data collection on intraoperative bleeding and the frequency of hemostasis during ESD. Further prospective research is necessary to clarify these matters.
In conclusion, CSS administration is ineffective in preventing post-colorectal ESD bleeding. Our results indicate the necessity to reconsider the application of CSS for preventing post-colorectal ESD bleeding.
Acknowledgment
We thank Misaki Katakura for the assistance with the data input.
Statement of Ethics
This study underwent a centralized review by the Ethics Committee of Asahikawa Medical University and received approval from each participating institution with the approval number C22098. Opt-out informed consent protocol was used for use of participant data for research purposes. This consent procedure was centrally reviewed by the Ethics Committee of Asahikawa Medical University and obtained approval from each participating institution under the approval number C22098 on December 14, 2022. We retrospectively reviewed anonymized clinical data after each patient received standard management. Individuals cannot be identified based on the data presented.
Conflict of Interest Statement
Mikihiro Fujiya received lecture fees from Olympus Corporation and Fujifilm Corporation. Keitaro Takahashi, Takuya Iwama, Kazuyuki Tanaka, Yuki Miyazawa, Shohei Kuroda, Masashi Horiuchi, Seisuke Saito, Momotaro Muto, Aki Sakatani, Katsuyoshi Ando, Nobuhiro Ueno, Shin Kashima, Kentaro Moriichi, Hiroki Tanabe, and Toshikatsu Okumura have no conflicts of interest or financial ties to disclose.
Funding Sources
The authors have received no funding.
Author Contributions
K.T. and M.F. conducted the study and wrote the initial draft of the manuscript. T.I., K.T., Y.M., S.K., M.H., S.S., M.M., and A.S. have contributed to data collection at each institution. K.A., N.U., and S.K. contributed to the analysis and interpretation of the data. K.M., H.T., and T.O. have critically reviewed the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding Statement
The authors have received no funding.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants, but are available from the corresponding author, K.T., upon reasonable request.
Supplementary Material
References
- 1. Oka S, Tanaka S, Saito Y, Iishi H, Kudo S, Ikematsu H, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015;110(5):697–707. [DOI] [PubMed] [Google Scholar]
- 2. Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81(3):583–95. [DOI] [PubMed] [Google Scholar]
- 3. Saito Y, Yamada M, So E, Abe S, Sakamoto T, Nakajima T, et al. Colorectal endoscopic submucosal dissection: technical advantages compared to endoscopic mucosal resection and minimally invasive surgery. Dig Endosc. 2014;26(Suppl 1):52–61. [DOI] [PubMed] [Google Scholar]
- 4. Imai K, Hotta K, Ito S, Yamaguchi Y, Kishida Y, Yabuuchi Y, et al. A risk‐prediction model for en bloc resection failure or perforation during endoscopic submucosal dissection of colorectal neoplasms. Dig Endosc. 2020;32(6):932–9. [DOI] [PubMed] [Google Scholar]
- 5. Libânio D, Pimentel-Nunes P, Bastiaansen B, Bisschops R, Bourke MJ, Deprez PH, et al. Endoscopic submucosal dissection techniques and technology: European society of gastrointestinal endoscopy (ESGE) technical review. Endoscopy. 2023;55(4):361–89. [DOI] [PubMed] [Google Scholar]
- 6. Li R, Cai S, Sun D, Shi Q, Ren Z, Qi Z, et al. Risk factors for delayed bleeding after endoscopic submucosal dissection of colorectal tumors. Surg Endosc. 2021;35(12):6583–90. [DOI] [PubMed] [Google Scholar]
- 7. Miyakawa A, Kuwai T, Sakuma Y, Kubota M, Nakamura A, Itobayashi E, et al. The efficacy of prophylactic clip closure of mucosal defects after colorectal endoscopic submucosal dissection on delayed bleeding. Scand J Gastroenterol. 2021;56(10):1236–42. [DOI] [PubMed] [Google Scholar]
- 8. Omori J, Goto O, Habu T, Ishikawa Y, Kirita K, Koizumi E, et al. Prophylactic clip closure for mucosal defects is associated with reduced adverse events after colorectal endoscopic submucosal dissection: a propensity-score matching analysis. BMC Gastroenterol. 2022;22(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogiyama H, Tsutsui S, Murayama Y, Maeda S, Satake S, Nasu A, et al. Prophylactic clip closure may reduce the risk of delayed bleeding after colorectal endoscopic submucosal dissection. Endosc Int Open. 2018;6(5):E582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobara H, Tada N, Fujihara S, Nishiyama N, Masaki T. Clinical and technical outcomes of endoscopic closure of postendoscopic submucosal dissection defects: literature review over one decade. Dig Endosc. 2023;35(2):216–31. [DOI] [PubMed] [Google Scholar]
- 11. Miyamoto Y, Ohbe H, Ishimaru M, Matsui H, Fushimi K, Yasunaga H. The effect of carbazochrome sodium sulfonate in patients with colonic diverticular bleeding: propensity score matching analyses using a nationwide inpatient database. Intern Med. 2020;59(15):1789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo Y, Zhao X, Releken Y, Yang Z, Pei F, Kang P. Hemostatic and anti-inflammatory effects of carbazochrome sodium sulfonate in patients undergoing total knee arthroplasty: a randomized controlled trial. J Arthroplasty. 2020;35(1):61–8. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi K, Sasaki T, Ueno N, Uehara K, Kobayashi Y, Sugiyama Y, et al. Carbazochrome sodium sulfonate is not effective for prevention of post-gastric endoscopic submucosal dissection bleeding: a retrospective study. Surg Endosc. 2022;36(10):7486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato M, Uedo N, Hokimoto S, Ieko M, Higuchi K, Murakami K, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment: 2017 appendix on anticoagulants including direct oral anticoagulants. Dig Endosc. 2018;30(4):433–40. [DOI] [PubMed] [Google Scholar]
- 15. Fujimoto K, Fujishiro M, Kato M, Higuchi K, Iwakiri R, Sakamoto C, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26(1):1–14. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32(2):219–39. [DOI] [PubMed] [Google Scholar]
- 17. Luo Y, Releken Y, Yang D, Yue Y, Liu Z, Kang P. Effects of carbazochrome sodium sulfonate combined with tranexamic acid on hemostasis and inflammation during perioperative period of total hip arthroplasty: a randomized controlled trial. Orthop Traumatol Surg Res. 2022;108(1):103092. [DOI] [PubMed] [Google Scholar]
- 18. Sugimoto M, Hatta W, Tsuji Y, Yoshio T, Yabuuchi Y, Hoteya S, et al. Risk factors for bleeding after endoscopic submucosal dissection for gastric cancer in elderly patients older than 80 Years in Japan. Clin Transl Gastroenterol. 2021;12(9):e00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okamoto Y, Oka S, Tanaka S, Inagaki K, Tanaka H, Matsumoto K, et al. Clinical usefulness of the S-O clip during colorectal endoscopic submucosal dissection in difficult-to-access submucosal layer. Endosc Int Open. 2020;8(3):E437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiba H, Ohata K, Tachikawa J, Arimoto J, Ashikari K, Kuwabara H, et al. Delayed bleeding after colorectal endoscopic submucosal dissection: when is emergency colonoscopy needed? Dig Dis Sci. 2019;64(3):880–7. [DOI] [PubMed] [Google Scholar]
- 21. Terasaki M, Tanaka S, Shigita K, Asayama N, Nishiyama S, Hayashi N, et al. Risk factors for delayed bleeding after endoscopic submucosal dissection for colorectal neoplasms. Int J Colorectal Dis. 2014;29(7):877–82. [DOI] [PubMed] [Google Scholar]
- 22. Seo M, Song EM, Cho JW, Lee YJ, Lee B-I, Kim JS, et al. A risk-scoring model for the prediction of delayed bleeding after colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2019;89(5):990–8.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants, but are available from the corresponding author, K.T., upon reasonable request.