Abstract
Background:
Emotional dysregulation affects up to two-thirds of adult patients with attention-deficit/hyperactivity disorder (ADHD) and is increasingly seen as a core ADHD symptom that is clinically associated with greater functional impairment and psychiatric comorbidity. We sought to investigate emotional dysregulation in ADHD and explored its neural underpinnings.
Methods:
We studied emotion induction and regulation in a clinical cohort of adult patients with ADHD before and after a stimulant challenge. We compared patients with age- and gender-matched healthy controls using behavioural, structural, and functional measures. We hypothesized that patients would demonstrate aberrant emotion processing compared with healthy controls, and sought to find whether this could be normalized by stimulant medication.
Results:
Behaviourally, the ADHD group showed reduced emotion induction and regulation capacity. Brain imaging revealed abberant activation and deactivation patterns during emotion regulation, lower grey-matter volume in limbic and paralimbic areas, and greater grey-matter volume in visual and cerebellar areas, compared with healthy controls. The behavioural and functional deficits seen in emotion induction and regulation in the ADHD group were not normalized by stimulant medication.
Conclusion:
Patients with ADHD may have impaired emotion induction and emotion regulation capacity, but these deficits are not reversed by stimulant medication. These results have important clinical implications when assessing which aspects of emotional dysregulation are relevant for patients and if and how traditional ADHD pharmacotherapy affects emotion induction and emotion regulation.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder that persists later in life and affects around 2.5% of adults.1,2 The core symptoms of ADHD include inattention and impulsivity or hyperactivity; these symptoms occur either in isolation or in combination to form different subtypes.3 Up to two-thirds of patients with ADHD are considered to have deficits in emotional processing capacities.4,5 Emotional dysregulation, although not formally a part of the diagnostic criteria for ADHD, is nonetheless considered by many to be a core feature of ADHD, related to a more severe clinical presentation, higher risk for psychiatric comorbidities, and poor functional outcomes.6–9
The stages of emotional information processing proceed in a loop from emotion perception, emotion induction (EI), automatic emotion regulation (ER), effortful ER, and, finally, to expressive behaviour.10 Emotional stimuli are salient and command attention in a valence-dependent manner.11,12 There is evidence that this process may be altered among patients with ADHD,13,14 with some experimental neuroimaging studies implicating the amygdala and other limbic and paralimbic abnormalities.15–17 Clinically, ADHD is associated with aberrant emotion generation, characterized by impulsive, as well as fast-rising and heightened emotion reactivity, giving rise to emotional lability.18 Moreover, experimental studies have revealed different facets of EI impairment in ADHD. For example, recent studies have demonstrated emotion recognition deficits in ADHD, with slower and less accurate response to emotional (but not to nonemotional) stimuli.19,20 Implicit, automatic ER is also thought to be altered.21 Cognitive control of emotion (i.e., voluntary and effortful ER) is necessary for flexible and adaptive emotion management and is fundamental for optimal daily functioning and good mental health. Clinically, patients with ADHD are thought to experience deficits in this domain, with suboptimal self-regulation of the emotional experience and behaviour, including limited and unsuccessful use of reappraisal and preferential use of suppression, which is correlated with reduced connectivity of the amygdala–prefrontal cortex.22–25 In addition, a phenomenon of dyssynchrony between the emotional experience and behaviour has been described, namely expression of behaviour disproportionate in intensity and duration to the emotional experience.18
Although diverse emotion-related problems can occur in ADHD with psychiatric comorbidities, it is well established that emotional dysregulation is also primarily associated with ADHD itself.18,26 However, it is debated if emotional and nonemotional27 cognitive problems are related or independent facets of ADHD. More specifically, it is not known whether emotional dysregulation (a deficit in “hot” cognition) is secondary to the cardinal ADHD symptoms of inattention and impulsivity (deficits in “cold” cognition) or whether they are independent in origin.4 One of the reasons why it is of great importance to disambiguate this concerns the development of effective treatment. If emotional dysregulation were secondary to the cold cognitive impairments seen in ADHD, then when the latter is optimally treated, it would be expected that impairments in hot cognition would also normalize. However, working memory and ER are not strongly correlated.28 Previous studies have shown that traditional pharmacological treatment for ADHD (i.e., stimulants and atomoxetine) facilitate partial improvement and moderate effects for emotional dysregulation, but smaller efficacy compared with that observed for cold, core ADHD symptoms.14,18,29–31
During the past few decades, more studies have explored the neural correlates of ADHD, while emotional dysregulation in this patient population has been less researched. For example, a few studies have demonstrated aberrant functional connectivity among patients with ADHD, such as hyperconnectivity or failure to appropriately deactivate the default-mode network (DMN), a functional network of brain regions associated with mind-wandering that is temporally anti-correlated with task-positive networks during the resting state32 and during cognitive tasks.33–35 There is even some evidence that psychostimulants can reverse such suboptimal connectivity,36,37 but it is not clear if this is relevant in the context of ER. Furthermore, Materna and colleagues21 recently found increased activation in the ventral anterior cingulate cortex (ACC) during a negative EI and ER task. Although the authors did not find evidence of alteration in effortful ER with reappraisal, they concluded that this finding is likely associated with enhanced implicit regulation, which is related to an emotional hyperresponsivity described in patients with ADHD. Schulz and colleagues38 found that lisdexamfetamine-related reduction in ADHD symptoms was correlated with functional disconnection of the amygdala from inferior frontal regions, possibly reducing the emotional burden on cognitive functions. Furthermore, functional and structural changes in limbic and paralimbic areas involved in bottom–up and top–down procesess have been reported in ADHD4 and are probably relevant to deficits in emotional processing. Importantly, many studies on this topic have been conducted in pediatric populations, which limits the validity of extrapolating these results to adults, given the temporal and developmental aspects of ADHD.
In this study, we sought to investigate emotional dysregulation in ADHD and explore its neural underpinnings using voxel-based morphometry (VBM) and functional magnetic resonance imaging (fMRI). Among the various aspects of emotional processing, we chose to specifically focus on EI and effortful ER using a well-characterized experimental paradigm to compare patients with ADHD and healthy controls.21,39–41 Since such experimental paradigms are of unknown ecological validity in clinical settings, it is crucial to use well-defined neuropsychological constructs — reappraisal, in our case — that can offer valuable insights into specific aspects of emotional processing and potentially serve as tools in clinical settings. We believe that EI and effortful ER, constructs included in the Research Domain Criteria project,42 are well suited for such purposes. Based on clinical observations, we hypothesized that patients with ADHD would demonstrate aberrant emotional processing, with more intense EI and deficient ER. Moreover, since some aspects of emotional dysregulation seem to improve with the prescription of stimulants, while others do not, we used the same paradigm without any pharmacological treatment and repeated it after ingestion of ADHD treatment with stimulants, hypothesizing that this intervention would have no or small effects on emotional processing. In terms of emotional dysregulation, we hypothesized that, during this task, we would find hypoactivation of control regions and nodes of task-positive functional networks on par with hyperactivation of nodes of task-negative networks, such as the DMN.43,44 Finally, we sought to explore structural differences in the brain of patients with ADHD; based on previous research, we hypothesized that patients with ADHD would show structural changes in limbic and paralimbic areas.43,45,46
Methods
Participants
We recruited participants from the local community and outpatient clinic at Örebro University Hospital. We conducted an a priori power analysis based on a previous study with a similar experimental design, (Appendix 1, available at www.jpn.ca/lookup/doi/10.1503/jpn.240009/tab-related-content).40 All patients received their ADHD diagnoses as adults and responded to treatment. We also recruited age- and gender-matched healthy controls. All participants were able and willing to provide written informed consent and were at least 18 years of age at the time of recruitment. The inclusion criteria for controls required that they be free of any psychiatric, neurologic, and addiction disorders; current drug use, including psychoactive medication; and any contraindication for methylphenidate. We asked all participants to abstain from alcohol consumption at least 1 day before the trial and instructed them to continue their usual consumption of coffee and nicotine and to keep it at the same level before each part of the testing.
Study design
The experiment consisted of a counterbalanced block design to examine the EI and ER of negative emotions before and 1–2 hours after ingestion of short-acting methylphenidate (30 mg) for healthy controls or methylphenidate or lisdexamphetamine as selected and dose-optimized by the treating physician for patients. We instructed patients to abstain from their ADHD medication for 24 hours beforehand.
The task consisted of 6 blocks of stimuli, with each block lasting 30 seconds, preceded and followed by a 30-second period of rest. In each block, we presented 5 pictures with negative emotional valence, each for 6 seconds, followed by a 2-second interval to separate them from each other. The 6 blocks were randomly counterbalanced to 1 of 2 conditions, EI or ER, with each condition repeated 3 times, thus allowing the functional response to be disentangled from physiologic confounds. During rest, the screen displayed either the word “feel” or “regulate” on a white background, denoting the ensuing pictures as an EI or ER block. Participants were told that when the word “feel” appeared, they were to simply look at the pictures allowing them to induce any emotional response, but when the word “regulate” appeared, to try to reduce the intensity of emotion the pictures generated by cognitively reapprasing their importance. In addition, we gave participants examples of reappraisal and instructed them to not use other ER methods, such as suppression. To reduce carry-over and anticipatory effects, we randomized the order of conditions, as well as the order of the stimuli within each block. The tasks were implemented in E-Prime (version 2.1, http://www.pstnet.com/eprime.cfm).
We used the International Affective Picture System (IAPS)47 as a source of the standardized pictures for the tasks (Appendix 1). As described above, the participants were instructed to either passively view the ensuing pictures (EI) or to actively downregulate the emotion using reappraisal (ER). To ascertain that they conformed to the instructions given to them, we interviewed the participants immediately after the the experiment as to the specific strategy they used, which we documented verbatim.
Data acquisition
At the end of the experiments, immediately after viewing the images, we asked participants to score emotion intensity for every image on a scale ranging from 1 to 9, where 1 represented the lowest level of emotion intensity and 9 the highest level. We calculated the sum score for the images to represent the emotion rating for each condition (EI and ER). We calculated ER capacity as the difference between emotion intensity during ER and EI (ER score = emotion intensity during EI – emotion intensity during ER). For the analysis of behavioural data, we employed mixed-effect, linear regression models and performed statistical analysis using Stata 14 software (StataCorp).
We collected neuroimaging data using a GE SIGNA Premier 3 T MRI scanner. We acquired T1 anatomic images using a sagittal 3-dimensional fast-spoiled gradient-echo sequence (repetition time 8.1 ms, echo time 3.1 ms, flip angle 12°, field of view 256 mm, voxel size 1 × 1 × 1 mm3). We collected fMRI data using a gradient-echo echo-planar imaging sequence (repetition time 2000 ms, echo time 30 ms, flip angle 80°, field of view 240 mm, voxel size 1.875 × 18.75 × 2 mm3). Two independent sessions were acquired per participant, one before medication and another with medication.
We used fMRIPrep software (version 22.0.2) to preprocess the fMRI data.48 The preprocessing steps included slice timing correction, head motion estimation, brain extraction, and co-registration to the Montreal Neurological Institute’s (MNI) MNI152NLin2009cAsym template with spatial normalization to a template resolution (isotropic voxel size of 2 mm). In addition to the fMRIPrep pipeline, we performed additional data scaling and covariate removal steps. The fMRI data were scaled to z scores using the mean and standard deviation of the entire fMRI time series. We removed covariates from the fMRI data, namely motion correction translation and rotation parameters, mean cerebrospinal fluid signal, and mean white-matter signal. We performed these data scaling and covariate removal steps using AFNI (version 23, https://afni.nimh.nih.gov/) programs (3dTstat, 3dcalc, and 3dDeconvolve).
Statistical analysis
For both first- and second-level statistical analysis, we employed the FSL FEAT pipeline. We chose a full-model setup and explored a number of contrasts (EI > ER, ER > EI, deactivation during EI, deactivation during ER, pre > post and post > pre for both EI and ER, controls > patients and patients > controls for both EI and ER). We reported MNI coordinates of statistically significant activation clusters that exceeded a threshold of Z greater than 3.1 and a (corrected) cluster significance level of p less than 0.05. We used the Benjamini–Hochberg procedure (false discovery rate = 0.05) to correct for multiple comparisons. To find the significant percentage of activated areas in the brain, we used the Harvard–Oxford Cortical and Subcortical Structural Atlas.
We used FSL-VBM software (version 1.1) to perform a VBM analysis on the T1-weighted structural MRI data.49 The preprocessing steps included brain extraction, tissue segmentation, spatial normalization to the MNI’s MNI152NLin2009cAsym template, and modulation by the Jacobian determinant of the deformation field. The resulting images of grey matter, white matter, and cerebrospinal fluid were smoothed with a Gaussian kernel at a full width at half-maximum of 7 mm. We performed permutation-based nonparametric inference using threshold-free cluster enhancement.
Ethics approval
The study was approved by the Stockholm County’s ethics committee (Dnr 2020–02278 and 2020–05590). Before the experiments, we provided participants with an overview of the general scope of the study and the outline of the experimental procedure. All participants gave written informed consent before the start of the experiment, in accordance with the Declaration of Helsinki.
Results
Behavioural data
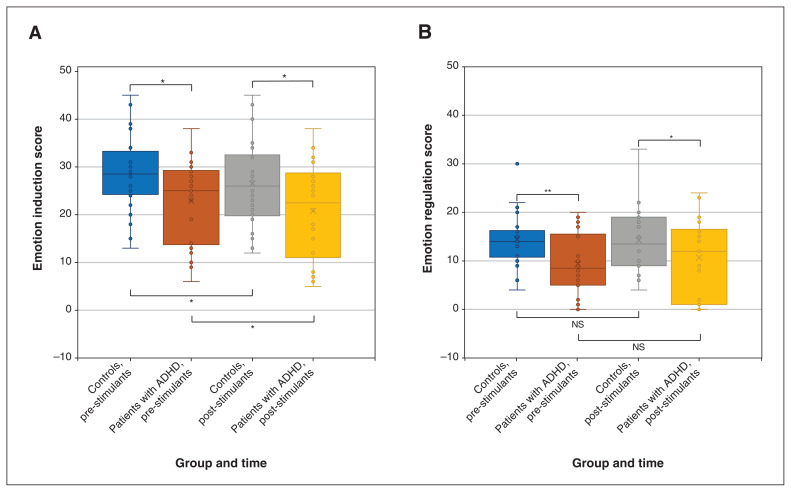
We collected behavioural data from 24 patients with ADHD and 22 controls. A 2-factor (group × medication) linear mixed model showed a significant main effect for group and the group × medication interaction. Patients with ADHD had significantly lower EI scores before (mean difference 5.55, standard error [SE] 2.61, p = 0.03) and after (mean difference 5.75, SE 2.61, p = 0.03) stimulant medication, compared with healthy controls. Among both patients with ADHD (mean difference 2.33, SE 1.02, p = 0.02) and healthy controls (mean difference 2.13, SE 1.07, p = 0.04), stimulant medication slightly but significantly reduced EI compared with control conditions (Figure 1A).
Figure 1.
(A) Emotion induction (all comparions p < 0.05) and (B) emotion regulation scores among healthy controls and patients with attention-deficity/hyperactivity disorder (ADHD) before and after ingestion of either short-acting methylphenidate (controls) or methylphenidate or lisdexamphetamine (patients with ADHD) (controls v. ADHD pre-stimulants p < 0.01; controls v. ADHD post-stimulants p < 0.05; controls pre- v. post-stimulants p > 0.05; ADHD pre- v. post-stimulants p > 0.05). Note: NS = nonsignificant. *p < 0.05, **p < 0.01.
A 2-factor (group × medication) linear mixed model showed a significant main effect for group and for the group × medication interaction. Patients with ADHD had significantly lower ER capacity scores before (mean difference 5.33, SE 1.91, p = 0.005) and after (mean difference 3.89, SE 1.91, p = 0.04) stimulant medication, compared with controls. We also investigated the medication effect on ER per se and found no significant effect among patients with ADHD (p = 0.2) or controls (p = 0.9; Figure 1B).
Functional MRI
We saw no specific task-related differences in activation in emotion-generating or emotion-regulating areas of the brain before and after ingestion of stimulant medication for either controls or patients with ADHD.
Between-group comparison of controls (n = 24) and patients (n = 28) showed no significant results. However, since we saw no effects of the pharmacological interventions, we performed a secondary exploratory analysis removing this covariate (total n = 48 controls, n = 56 patients with ADHD).
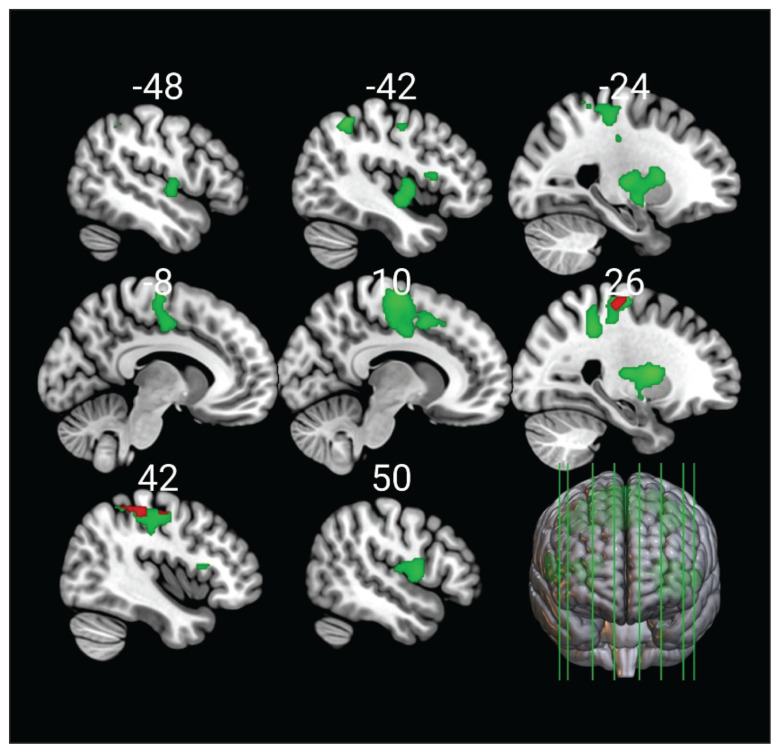
We found that during EI, the contrast of controls to patients with ADHD revealed significant activation in the frontal (precentral gyrus, supplementary motor area, superior frontal gyrus) and parietal areas (superior parietal lobule, postcentral gyrus, supramarginal gyrus) (Figure 2). During ER, this contrast showed significant activations in a widely distributed neural network that encompassed 6 large clusters including the frontal (precentral gyrus, supplementary motor area, inferior and superior frontal gyrus), temporal (Heschl gyrus), parietal (postcentral gyrus, superior parietal lobule, supramarginal gyrus, angular gyrus), and occipital areas (lateral occipital cortex), as well as the cingulate cortex (anterior and posterior parts), insula and operculum, planum polare, and the putamen (Figure 2). The contrast of patients with ADHD to controls did not show any significant results.
Figure 2.
Main effects for emotion induction (red) and emotion regulation (green) among healthy controls in contrast to patients with attention-deficit/hyperactivity disorder (ADHD). There were no significant activations among patients with ADHD in contrast to healthy controls. Analysis was conducted without stimulant medication as a covariate.
Healthy controls showed significantly greater activation in the right midle frontal gyrus during ER than EI. No areas had greater activation during EI among controls. Patients with ADHD had greater activation of the left thalamus, cerebellum, and occipital lobe during EI than ER. No areas had greater activation during ER among patients with ADHD.
Focusing on areas that were deactivated during ER, we found that the posterior cingulate cortex and the precuneus were deactivated among healthy controls, whereas, for patients with ADHD, we observed widespread deactivations including the posterior cingulate cortex, the precuneus, the operculum, the insular cortex, Heschl gyrus, and the precentral gyrus.
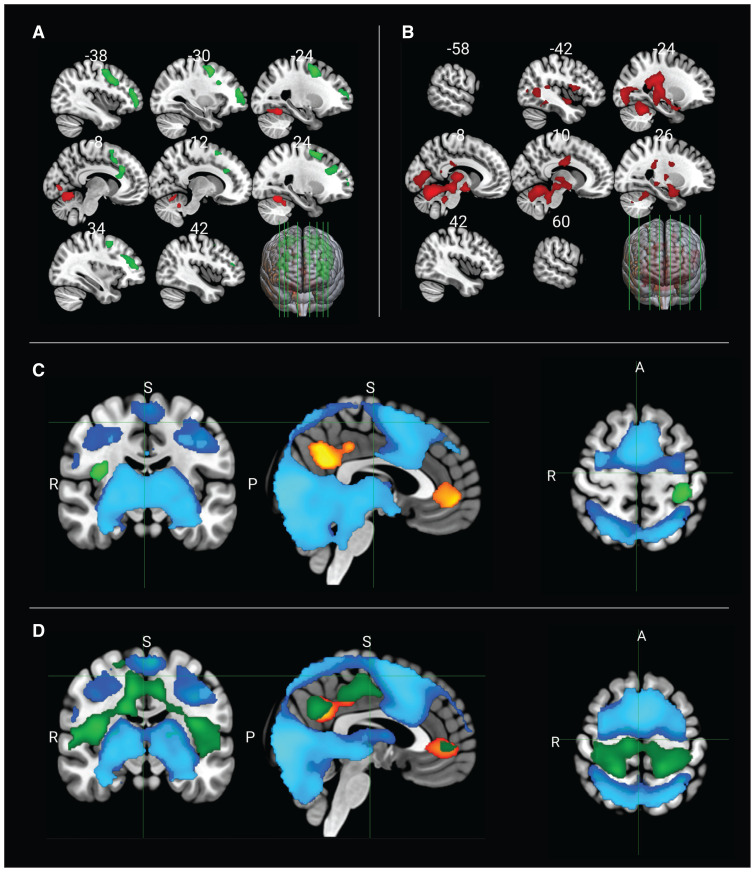
Again, since we saw no effects of the pharmacological interventions, we performed a post hoc exploratory analysis removing the stimulant covariate. The contrast of EI to ER among controls showed activations in areas including the precuneus, the temporal occipital fusiform cortex, the lingual gyrus, and the intra- and supracalcarine cortex (primary visual cortex) (Figure 3A). We then specifically focused on the areas that showed significant deactivation during EI; these were located in the precuneus cortex, the posterior cingulate cortex, the paracingulate gyrus, the superior and middle frontal gyrus, the frontal medial cortex, the ACC, the frontal pole, and the caudate nucleus (Figure 3C).
Figure 3.
Main effects for emotion induction in contrast to emotion regulation (red) and emotion regulation in contrast to emotion induction (green) among (A) healthy controls and (B) patients with attention-deficit/hyperactivity disorder (ADHD). (C) Significant activations and deactivations for emotion induction and (D) emotion regulation among controls and patients with ADHD (dark blue = activation among controls; light blue = activation among patients with ADHD; orange–yellow = deactivation among controls; green = deactivation among patients with ADHD). Analysis conducted without medication status as a covariate. Note: A = anterior, P = posterior, R = right, S = superior.
For patients with ADHD, the contrast of EI to ER showed activations in the thalamus, the cerebellum, and the parahippocampal gyrus (Figure 3B), while deactivations during EI were located in the pre- and postcentral gyri, the superior parietal lobule, the cental and parietal operculum, and the insula (Figure 3C).
The contrast of ER to EI among healthy controls revealed activations occupying the frontal pole, the superior and middle frontal gyrus, the precentral gyrus, the supplementary motor area, the ACC, and the paracingulate gyrus (Figure 3A). Areas located in the precuneus, the lingual cortex, the paracingulate gyrus, the frontal medial cortex, the frontal pole, and the cerebellum showed significant deactivation during ER among controls (Figure 3D).
Among patients with ADHD, we found no significant activations for the contrast of ER to EI (Figure 3B). Broad areas located in the operculum (parietal, central), the insula, Heschl gyrus, the fusiform gyrus, the lingual gyrus, the ACC, and the paracingulate gyrus were deactivated during ER among patients with ADHD (Figure 3D).
Voxel-based morphometry
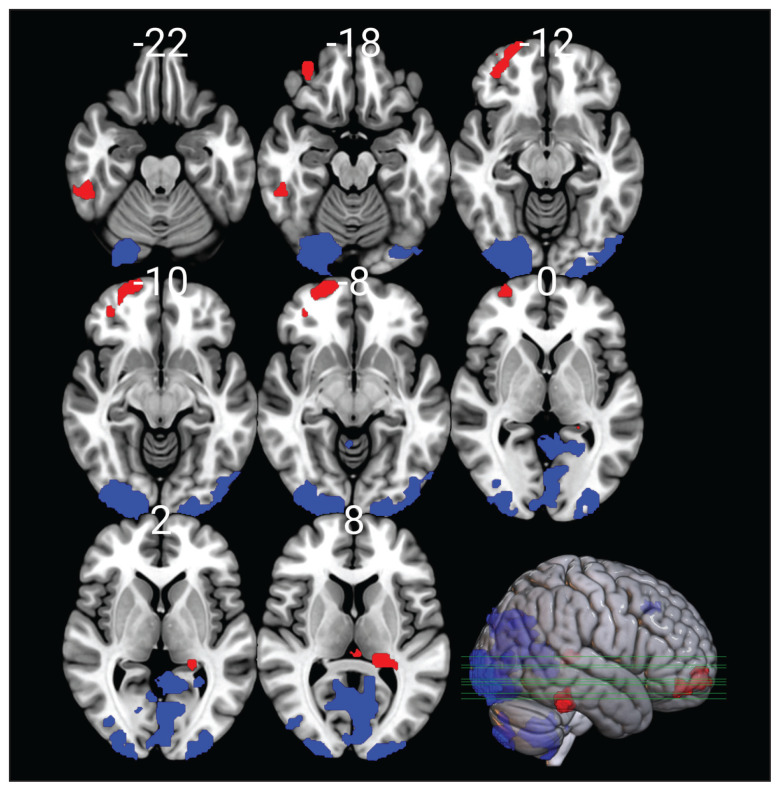
Medication status was not associated with any difference in grey-matter volume. Compared with healthy controls (n = 46), patients with ADHD (n = 62) had significantly lower (corrected p < 0.05) grey-matter volume in the limbic and paralimbic areas, more specifically, in the right frontal pole and frontal orbital cortex, the left thalamus, the left hippocampus, and the right fusiform gyrus and inferior temporal gyrus. Patients with ADHD, on the other hand, had significantly higher (corrected p < 0.05) grey-matter volume in visual areas (primary visual cortex, second or third visual area, middle occipital gyrus), the bilateral paracingulate gyrus, and the cerebellum (Figure 4 and Appendix 1).
Figure 4.
Voxel-based morphometric images showing brain areas where patients with attention-deficit/hyperactivity disorder had significantly lower (red) or higher (blue) grey-matter volume than healthy controls, after correction for multiple comparison.
Discussion
Emotional dysregulation in ADHD is increasingly acknowledged as a core feature of the disorder, separate from the problems with executive function and neuropsychological deficits.5,26,50 Its importance is highlighted by the fact that it is correlated with worse functional outcomes and comorbidities.4,18,51 Different phenotypes of emotional dysregulation — such as problems in emotion generation, ER, and expression in behaviour — may exist,18 but it is not well understood which aspects of the emotion processing continuum are most relevant in ADHD,10 whether they are amendable to stimulant medications,14,18,29–31 and how they are related to structural45,46 and functional4,32,43 brain abnormalities seen among patients with ADHD.
We employed a previously studied paradigm,39,40 whereby we used negatively valenced emotional pictures from the IAPS as a probe to gauge subjective and neural correlates to EI and effortful ER with cognitive reappraisal, in conjunction with structural and functional brain imaging, in the presence and absence of psychostimulants used for the treatment of ADHD.
Behaviourally, contrary to our hypothesis, patients with ADHD scored emotion intensity, as induced by the pictures when passively looking at them, significantly lower than healthy controls. Our findings also contrasted with the increased emotional reactivity in this patient group that has been described clinically.18 This mismatch between experimental findings and clinical observations is not well understood and needs further exploration. It is possible, for example, that participants reported low intensity in emotional experience under the controlled research settings, as this was implicitly compared with a baseline of generally increased emotionality. Moreover, during EI, patients with ADHD displayed increased activity in sensory processing areas, such as the thalamus and the occipital cortex, compared with ER. However, the emotional experience is thought to be the sum product of emotion generation and implicit automatic ER. Consistent with our results, previous experimental studies have demonstrated slower and less accurate response to emotional stimuli19,20 and increased implicit ER21 in this patient group. Thus, another perspective congruent to our findings of reduced EI and to compromised emotion recognition, observed elsewhere,19,20 is the association with alexithymia that seems to be more prevalent among patients with ADHD than the general population; alexithymia is also correlated with other symptoms such as anxiety and impulsivity.52,53 Moreover, yet another perspective to be explored is the temporal and developmental aspects of emotional dysregulation in ADHD, as it is possible that the fast-rising, increased emotionality could be a phenotype that is mostly seen in children and normalized to some extent later in life, possibly because of reactive implicit ER processes.21
Given these results, clinicians and future studies should not limit characterization of deficits of emotion generation within the notion of explosive emotion reactivity but rather consider that hindered EI and generation can also be a part of the psychopathology in ADHD. This interpretation is crucial as, under the experimental conditions of this study, both control participants and patients with ADHD reported reduced emotional intensity after intake of psychostimulants. Extrapolating these results, the outcome of pharmacological treatment with stimulants on EI can be viewed as positive if overly intense emotions are seen as a part of the pathological presentation, but if alexithymia and deficient emotion recognition mediate the reported emotional lability and impulsivity, then further decreasing emotion intensity with medication could be an undesirable, counterproductive effect.
Furthermore, as hypothesized, patients with ADHD demonstrated less capacity for ER when using cognitive reappraisal for negative emotions, but also showed different brain activation patterns. One of the notable differences was that, when regulating, patients with ADHD failed to preferentially engage prefrontal control areas compared with baseline EI, as healthy controls did. Direct comparison between healthy controls and patients with ADHD also showed greater activation for the former group, including several frontal and parietal areas relevant to task-positive attention networks — such as the dorsal attention network, the ventral attention network, and the frontoparietal network — as well as areas relevant to response inhibition, such as the inferior frontal gyrus and the insula. Moreover, we chose to contrast negative emotions to a nonspecific baseline to be able to assess not only differences in task-induced activations, but also differences in task-induced deactivations. Indeed, it is interesting to observe that, during active ER, healthy controls showed deactivation in areas related to the DMN, whereas patients with ADHD showed more widespread deactivation covering sensory areas such as visual (fusiform gyrus, lingual gyrus) and auditory areas (Heschl gyrus), as well as the operculum, insula, and the anterior cingulum, with the latter also part of the salience and the cingulo-opercular network. It is well documented that patients with ADHD preferentially use suppression as an ER strategy, which comes with greater processing costs and is less efficient, rather than cognitive reappraisal.25,51 Excessive mind-wandering is a mechanism proposed to interfere in tasks that demand cognitive control and mediate decline in performance in nonemotional settings;4 this may be mediated by an inability to efficiently deactivate the DMN in favour of activating task-positive functional networks.32,43 Indeed, our findings suggest that, even for a cognitive task involving emotional context, patients with ADHD show subefficient deactivation of the DMN during the task of ER, compared with healthy controls. Instead, patients with ADHD further deactivated sensory areas and nodes of the saliency and cingulo-opercular network that are related to assessment of the homeostatic relevance of external stimuli and maintenance of tonic alertness during executive control.54,55
Importantly, the capacity for ER with reappraisal was not significantly affected by stimulants in either the control or ADHD groups, in behavioural terms. In parallel, we did not observe any normalization or other significant change in aberrant functional activity.
Finally, we found structural differences in ADHD, with reduced grey-matter volumes in the limbic and paralimbic areas, more specifically in the areas in the frontal pole covering the ventromedial and ventrolateral areas, the right thalamus, and the hippocampus and left fusiform gyrus. The involvement of the most rostral prefrontal regions in emotion processing is well studied,56 as their rich reciprocal connections with other subcortical regions and the lateral cortex place it optimally to mediate the regulation of affective experiences.57,58 According to Damasio’s somatic marker hypothesis,59–61 patients with a suboptimally functioning ventromedial prefrontal cortex may experience difficulty making use of emotion-related somatic markers and thus exhibit emotion and behaviour regulation problems, a framework relevant for dampened emotion generation, alexithymia, and emotional dysregulation. There is also a rich literature associating the right inferior frontal areas with inhibiting response tendencies and processes of cognitive control.62,63 Moreover, the role of the hippocampus in emotion processing — with increased local activation and increased functional coupling with the amygdala in emotional contexts — is well documented.64,65 For example, patients with hippocampal damage have demonstrated difficulty in retrieving fear associations to contextual cues, although these associations may have been implicitly learned correctly.66 Finally, we found differences in the fusiform gyrus, a part of the visual–limbic circuit67,68 that is activated in response to face perception,69 as well as to negative stimuli such as disgust,70 and an area whose aberrant activity has been implicated in other affective disorders.71,72 Hence, the structural changes seen in this study may be contributing to the deficit in emotion generation and recognition seen here and in other studies. Although changes in these areas have been previously described,45,46 this specific constellation is not expected to be found across all cases and, therefore, statistical significance is not guaranteed in large studies with less homogeneous cohorts.19,20
Limitations
This multimodal study employed behavioural measures, as well as functional and structural neuroimaging, and included both patients with ADHD and healthy participants before and after use of psychostimulant medication. We used this multifaceted and powerful setup to explore different aspects of emotional processing. However, the sample size and our cross-sectional study design limit the generalizability of our findings and their extrapolation to the clinical setting. Larger samples sizes and longitudinal studies that track changes in brain activity over time and compare these changes among treated and untreated people with ADHD could provide stronger evidence. Moreover, our study did not include a placebo control group, which means that we cannot rule out the possibility that some of the observed changes were owing to nonspecific effects of taking a medication or repetition of the task. Future studies that include a placebo control group would help to address this limitation. Another limitation of our study is related to the paradigm used for fMRI data acquisition. Our task paradigm included only visual stimuli, which may have disproportionately recruited the visual areas of the brain. As a result, contrasting any task to rest may have primarily captured changes in visual processing, rather than the specific effects of psychostimulants on ER, attentional control, and reward processing. Future studies could include task paradigms that target a broader range of cognitive processes and sensory modalities, such as auditory or somatosensory stimuli. A simple remedy to limit visual cortex activation would be to replace the blank screen displayed during rest periods of the paradigm with emotionally neutral pictures. Another important aspect to consider relates differences in assessment of emotional response, either directly after stimulus presentation21 or, as in our case, post hoc after the end of the session. We chose the latter strategy to minimize interference from the assessment per se, as the very act of appraising, naming, and labelling emotions sets in motion implicit ER processes. This approach carries instead greater risk for interdependence in the rating of the different pictures and inserts a memory component into the affective assessment, which may partly explain the differences between our results and those of Materna and colleagues.21 Moreover, our decision to contrast negative emotions with a stimulus-free baseline carries the drawback of including nonspecific processes unrelated to emotional salience.
Conclusion
We employed a negative EI and ER paradigm and found that patients with ADHD demonstrated lower capacity for EI and ER using cognitive reappraisal, compared with controls. In addition, our results showed a suboptimal deactivation of hubs of the DMN during the ER task periods among patients with ADHD. Ingestion of psychostimulants reduced EI scores for all participants, regardless of their diagnosis status, but affected neither the behavioural measures nor the deviant activation of task-negative functional networks during ER. We found structural changes in cortical and subcortical areas relevant to emotion processing that might be related to the aberrant EI and ER among patients with ADHD. These results have important clinical implications when assessing which aspects of emotional dysregulation are relevant for patients with ADHD and, critically, if and how traditional pharmacotherapy with stimulants affect EI and ER.
Footnotes
Competing interests: None declared.
Contributors: Mussie Msghina conceived the study, and all authors contributed to the design of the work. Per Thunberg and Mussie Msghina acquired the data. Myrto Sklivanioti Greenfield, Yanlu Wang, Paul Hamilton, and Mussie Msghina contributed to data analysis. All of the authors contributed to data interpretation. Myrto Sklivanioti Greenfield and Mussie Msghina drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding: Financial support was provided by ALF funding and Nyckelfonden (OLL - 787911, 93542) in Region Örebro County.
Data sharing: The raw data supporting the conclusions of this article will be made available by the authors on request, without undue reservation.
References
- 1.Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 2009;194:204–11. [DOI] [PubMed] [Google Scholar]
- 2.Fayyad J, Sampson NA, Hwang I, et al. The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. Atten Defic Hyperact Disord 2017;9:47–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth edition. Arlington (VA): American Psychiatric Association Publishing; 2013. [Google Scholar]
- 4.Shaw P, Stringaris A, Nigg J, et al. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry 2014;171:276–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surman CB, Biederman J, Spencer T, et al. Understanding deficient emotional self-regulation in adults with attention deficit hyperactivity disorder: a controlled study. Atten Defic Hyperact Disord 2013;5:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosello B, Berenguer C, Raga JM, et al. Executive functions, effortful control, and emotional lability in adults with ADHD: implications for functional outcomes. Psychiatry Res 2020;293:113375. [DOI] [PubMed] [Google Scholar]
- 7.Mayer JS, Brandt GA, Medda J, et al. Depressive symptoms in youth with ADHD: the role of impairments in cognitive emotion regulation. Eur Arch Psychiatry Clin Neurosci 2022;272:793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanabra M, Gómez-Hinojosa T, Grau N, et al. Deficient emotional self-regulation and sleep problems in ADHD with and without pharmacological treatment. J Atten Disord 2022;26:426–33. [DOI] [PubMed] [Google Scholar]
- 9.Welkie J, Babinski DE, Neely KA. Sex and emotion regulation difficulties contribute to depression in young adults with attentiondeficit/hyperactivity disorder. Psychol Rep 2021;124:596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross JJ, Barrett LF. Emotion generation and emotion regulation: one or two depends on your point of view. Emot Rev 2011;3:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. J Exp Psychol Gen 2001;130:466–78. [DOI] [PubMed] [Google Scholar]
- 12.Adolphs R, Mlodinow L, Barrett LF. What is an emotion? Curr Biol 2019;29:R1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du J, Li J, Wang Y, et al. Event-related potentials in adolescents with combined ADHD and CD disorder: a single stimulus paradigm. Brain Cogn 2006;60:70–5. [DOI] [PubMed] [Google Scholar]
- 14.Williams LM, Hermens DF, Palmer D, et al. Misinterpreting emotional expressions in attention-deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects. Biol Psychiatry 2008;63:917–26. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KP, Bédard AC, Fan J, et al. Emotional bias of cognitive control in adults with childhood attention-deficit/hyperactivity disorder. Neuroimage Clin 2014;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajima-Pozo K, Yus M, Ruiz-Manrique G, et al. Amygdala abnormalities in adults with ADHD. J Atten Disord 2018;22:671–8. [DOI] [PubMed] [Google Scholar]
- 17.Viering T, Hoekstra PJ, Philipsen A, et al. Functional network topology of the right insula affects emotion dysregulation in hyperactive-impulsive attention-deficit/hyperactivity disorder. Sci Rep 2021;11:15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraone SV, Rostain AL, Blader J, et al. Practitioner review: emotional dysregulation in attention-deficit/hyperactivity disorder - implications for clinical recognition and intervention. J Child Psychol Psychiatry 2019;60:133–50. [DOI] [PubMed] [Google Scholar]
- 19.Viering T, Naaijen J, van Rooij D, et al. Amygdala reactivity and ventromedial prefrontal cortex coupling in the processing of emotional face stimuli in attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry 2022;31:1895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuberer A, Schwarz L, Kreifelts B, et al. Neural basis of impaired emotion recognition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2022;7:680–7. [DOI] [PubMed] [Google Scholar]
- 21.Materna L, Wiesner CD, Shushakova A, et al. Adult patients with ADHD differ from healthy controls in implicit, but not explicit, emotion regulation. J Psychiatry Neurosci 2019;44:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts W, Milich R, Barkley RA. Primary symptoms, diagnostic criteria, subtyping, and prevalence of ADHD. In: Barkley RA, editor. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York (NY): The Guilford Press; 2015:51–80. [Google Scholar]
- 23.Hagstrøm J, Maigaard K, Pagsberg AK, et al. Reappraisal is an effective emotion regulation strategy in children with Tourette syndrome and ADHD. J Behav Ther Exp Psychiatry 2020;68:101541. [DOI] [PubMed] [Google Scholar]
- 24.Musser ED, Galloway-Long HS, Frick PJ, et al. Emotion regulation and heterogeneity in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2013;52:163–171.e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Chen W, Preece DA, et al. Emotion dysregulation in adults with ADHD: the role of cognitive reappraisal and expressive suppression. J Affect Disord 2022;319:267–76. [DOI] [PubMed] [Google Scholar]
- 26.Skirrow C, Asherson P. Emotional lability, comorbidity and impairment in adults with attention-deficit hyperactivity disorder. J Affect Disord 2013;147:80–6. [DOI] [PubMed] [Google Scholar]
- 27.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci 2005;9:242–9. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser A, Reneman L, Lucassen PJ, et al. Targeting working memory to modify emotional reactivity in adult attention deficit hyperactivity disorder: a functional magnetic resonance imaging study. Brain Imaging Behav 2022;16:680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conzelmann A, Woidich E, Mucha RF, et al. Methylphenidate normalizes emotional processing in adult patients with attention-deficit/hyperactivity disorder: preliminary findings. Brain Res 2011;1381:159–66. [DOI] [PubMed] [Google Scholar]
- 30.Rösler M, Retz W, Fischer R, et al. Twenty-four-week treatment with extended release methylphenidate improves emotional symptoms in adult ADHD. World J Biol Psychiatry 2010;11:709–18. [DOI] [PubMed] [Google Scholar]
- 31.Reimherr FW, Williams ED, Strong RE, et al. A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry 2007;68:93–101. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy H, Skokauskas N, Mulligan A, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry 2013;70:1329–37. [DOI] [PubMed] [Google Scholar]
- 33.Wolf RC, Plichta MM, Sambataro F, et al. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Hum Brain Mapp 2009;30:2252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubia K, Cubillo A, Smith AB, et al. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp 2010;31:287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vloet TD, Gilsbach S, Neufang S, et al. Neural mechanisms of interference control and time discrimination in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:356–67. [PubMed] [Google Scholar]
- 36.Peterson BS, Potenza MN, Wang Z, et al. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry 2009;166:1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posner J, Nagel BJ, Maia TV, et al. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2011;50:828–37.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz KP, Krone B, Adler LA, et al. Lisdexamfetamine targets amygdala mechanisms that bias cognitive control in attentiondeficit/hyperactivity disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:686–93. [DOI] [PubMed] [Google Scholar]
- 39.Sklivanioti Greenfield M, Wang Y, Msghina M. Similarities and differences in the induction and regulation of the negative emotions fear and disgust: a functional near infrared spectroscopy study. Scand J Psychol 2022;63:581–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sklivanioti Greenfield M, Wang Y, Msghina M. Behavioral, cortical and autonomic effects of single-dose escitalopram on the induction and regulation of fear and disgust: comparison with single-session psychological emotion regulation with reappraisal. Front Psychiatry 2023;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochsner KN, Bunge SA, Gross JJ, et al. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci 2002;14:1215–29. [DOI] [PubMed] [Google Scholar]
- 42.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010;167:748–51. [DOI] [PubMed] [Google Scholar]
- 43.Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 2010;31:904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 2014;24:2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoogman M, Bralten J, Hibar DP, et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 2017;4:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoogman M, Muetzel R, Guimaraes JP, et al. Brain imaging of the cortex in adhd: a coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry 2019;176:531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville (FL);2008. [Google Scholar]
- 48.Esteban O, Ciric R, Finc K, et al. Analysis of task-based functional MRI data preprocessed with fMRIPrep. Nat Protoc 2020;15:2186–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007;130:2375–86. [DOI] [PubMed] [Google Scholar]
- 50.Gisbert L, Richarte V, Corrales M, et al. The relationship between neuropsychological deficits and emotional lability in adults with ADHD. J Atten Disord 2019;23:1514–25. [DOI] [PubMed] [Google Scholar]
- 51.Soler-Gutiérrez AM, Pérez-González JC, Mayas J. Evidence of emotion dysregulation as a core symptom of adult ADHD: a systematic review. PLoS One 2023;18:e0280131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiraz S, Sertçelik S, Erdogan Taycan S. The relationship between alexithymia and impulsiveness in adult attention deficit and hyperactivity disorder. [Eriskin Dikkat Eksikligi Hiperaktivite Bozuklugunda Aleksitimi ve Dürtüsellik Iliskisi.]. Turk Psikiyatri Derg 2021;32:109–17. doi: 10.5080/u23775. [DOI] [PubMed] [Google Scholar]
- 53.Edel MA, Rudel A, Hubert C, et al. Alexithymia, emotion processing and social anxiety in adults with ADHD. Eur J Med Res 2010;15:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newbold DJ, Gordon EM, Laumann TO, et al. Cingulo-opercular control network and disused motor circuits joined in standby mode. Proc Natl Acad Sci U S A 2021;118:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menon V, D’Esposito M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 2022;47:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge University Press; 2008. [Google Scholar]
- 57.Motzkin JC, Philippi CL, Wolf RC, et al. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry 1969;77:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fitzgerald JM, Kinney KL, Phan KL, et al. Distinct neural engagement during implicit and explicit regulation of negative stimuli. Neuropsychologia 2020;145:106675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 1996;351:1413–20. [DOI] [PubMed] [Google Scholar]
- 60.Damasio AR. Toward a neurobiology of emotion and feeling: operational concepts and hypotheses. Neuroscientist 1995;1:19–25. [Google Scholar]
- 61.Damasio H, Grabowski T, Frank R, et al. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 1994;264:1102–5. [DOI] [PubMed] [Google Scholar]
- 62.Sundermann B, Pfleiderer B. Functional connectivity profile of the human inferior frontal junction: involvement in a cognitive control network. BMC Neurosci 2012;13:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 2004;8:170–7. [DOI] [PubMed] [Google Scholar]
- 64.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci 2006;7:54–64. [DOI] [PubMed] [Google Scholar]
- 65.Faul L, Stjepanovic D, Stivers JM, et al. Proximal threats promote enhanced acquisition and persistence of reactive fear-learning circuits. Proc Natl Acad Sci U S A 2020;117:16678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav Neurosci 2005;119:677–86. [DOI] [PubMed] [Google Scholar]
- 67.Herrington JD, Taylor JM, Grupe DW, et al. Bidirectional communication between amygdala and fusiform gyrus during facial recognition. Neuroimage 2011;56:2348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris JS, Friston KJ, Büchel C, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 1998;121:47–57. [DOI] [PubMed] [Google Scholar]
- 69.Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci 2004;7:555–62. [DOI] [PubMed] [Google Scholar]
- 70.Radua J, Sarro S, Vigo T, et al. Common and specific brain responses to scenic emotional stimuli. Brain Struct Funct 2014; 219:1463–72. [DOI] [PubMed] [Google Scholar]
- 71.Groenewold NA, Opmeer EM, de Jonge P, et al. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev 2013;37:152–63. [DOI] [PubMed] [Google Scholar]
- 72.Ekman CJ, Petrovic P, Johansson AG, et al. A history of psychosis in bipolar disorder is associated with gray matter volume reduction. Schizophr Bull 2017;43:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]