Abstract
BACKGROUND:
Alterations in the buffering of intracellular Ca2+, for which myofilament proteins play a key role, have been shown to promote cardiac arrhythmia. It is interesting that although studies report atrial myofibrillar degradation in patients with persistent atrial fibrillation (persAF), the intracellular Ca2+ buffering profile in persAF remains obscure. Therefore, we aimed to investigate the intracellular buffering of Ca2+ and its potential arrhythmogenic role in persAF.
METHODS:
Transmembrane Ca2+ fluxes (patch-clamp) and intracellular Ca2+ signaling (fluo-3-acetoxymethyl ester) were recorded simultaneously in myocytes from right atrial biopsies of sinus rhythm (Ctrl) and patients with persAF, alongside human atrial subtype induced pluripotent stem cell–derived cardiac myocytes (iPSC-CMs). Protein levels were quantified by immunoblotting of human atrial tissue and induced pluripotent stem cell–derived cardiac myocytes. Mouse whole heart and atrial electrophysiology were measured on a Langendorff system.
RESULTS:
Cytosolic Ca2+ buffering was decreased in atrial myocytes of patients with persAF because of a depleted amount of Ca2+ buffers. In agreement, protein levels of selected Ca2+ binding myofilament proteins, including cTnC (cardiac troponin C), a major cytosolic Ca2+ buffer, were significantly lower in patients with persAF. Small interfering RNA (siRNA)–mediated knockdown of cTnC (si-cTNC) in atrial iPSC-CM phenocopied the reduced cytosolic Ca2+ buffering observed in persAF. Si-cTnC treated atrial iPSC-CM exhibited a higher predisposition to spontaneous Ca2+ release events and developed action potential alternans at low stimulation frequencies. Last, indirect reduction of cytosolic Ca2+ buffering using blebbistatin in an ex vivo mouse whole heart model increased vulnerability to tachypacing-induced atrial arrhythmia, validating the direct mechanistic link between impaired cytosolic Ca2+ buffering and atrial arrhythmogenesis.
CONCLUSIONS:
Our findings suggest that loss of myofilament proteins, particularly reduced cTnC protein levels, causes diminished cytosolic Ca2+ buffering in persAF, thereby potentiating the occurrence of spontaneous Ca2+ release events and atrial fibrillation susceptibility. Strategies targeting intracellular buffering may represent a promising therapeutic lead in persAF management.
Keywords: atrial fibrillation, atrial remodeling, calcium signaling, cardiac arrhythmias, electrophysiology, ion channels
Clinical Perspective.
What Is New?
Here we provide the first in-depth analysis of cytosolic Ca2+ buffering in human atrial cardiac myocytes.
We demonstrate that cytosolic Ca2+ buffering is reduced in persistent atrial fibrillation (persAF), which promotes the occurrence of arrhythmogenic Ca2+ waves and AF maintenance.
By showing that myofibrillar degradation, particularly reduced expression of cTnC (cardiac troponin C), is a major contributor to altered Ca2+ buffering in persAF, we provide a novel mechanistic link between contractile dysfunction and the proarrhythmic substrate in atrial cardiac myocytes from patients with persAF.
What Are the Clinical Implications?
Modulation of the intracellular Ca2+ buffering provides a novel target that could be exploited in the treatment of persAF.
Clinically approved Ca2+ sensitizers such as levosimendan or omecamtiv mecarbil and nutritional supplements like taurine and β-alanine, possessing buffering modulatory properties, could be valuable additions to currently available therapeutics used in persAF management.
Editorial, see p 560
Atrial fibrillation (AF) is the most frequent cardiac arrhythmia and is associated with increased mortality and morbidity.1 Despite recent advances in the understanding of the molecular mechanisms underlying AF pathophysiology, treatment remains challenging, particularly because of the self-promoting remodeling induced by AF.2
Altered intracellular Ca2+ handling is a key contributor to AF-associated remodeling.3–5 In healthy cardiac myocytes, Ca2+ enters the cell during systole through voltage-gated L-type Ca2+ channels (ICa,L) and induces a much larger Ca2+ release from the sarcoplasmic reticulum (SR) through SR Ca2+ release channels (ryanodine receptors, RyR2 [cardiac ryanodine receptor type 2]). The released Ca2+ binds to cTnC (cardiac troponin C) and induces contraction of myofilaments. During diastole, Ca2+ is pumped back into the SR by SERCA (SR Ca2+-ATPase) and is extruded from the cell predominantly by the Na+-Ca2+ exchanger (NCX). In AF, normal physiological Ca2+ cycling is disturbed, and RyR2 fails to remain closed during diastole.6–8 This leads to spontaneous diastolic Ca2+ releases (spontaneous Ca2+ release events [SCaEs]), including sparks and waves, which are thought to play a major role in AF initiation and maintenance.3,9
It is important to note that only about 1% of cytoplasmic Ca2+ is free, whereas the remainder is bound to cytoplasmic Ca2+ buffers.10 Therefore, it is not surprising that even minor changes in the Ca2+ binding properties of Ca2+ buffers may have a huge impact on the size and kinetics of free Ca2+ transients (CaT). In addition, altered Ca2+ buffering has been suggested to be involved in the propagation of arrhythmogenic Ca2+ waves.11,12
Because cTnC is one of the most important Ca2+ buffers,10,13 and AF is associated with severe contractile dysfunction and degradation of myofilament proteins,14–17 we hypothesize that Ca2+ buffering is reduced in atrial myocytes from patients with AF and that this could contribute to arrhythmogenesis in patients with AF.
In the present study, we quantified cytosolic Ca2+ buffering in atrial cardiac myocytes from right atrial samples of patients in sinus rhythm (control) and persistent AF (persAF). Using atrial subtype human induced pluripotent stem cell–derived cardiac myocytes (iPSC-CMs), we demonstrate that knockdown of cTnC leads to reduced Ca2+ buffering and increases incidence of SCaEs, thereby phenocopying the Ca2+ handling alterations observed in patients with AF.3,4,7,8 Last, we use a new ex vivo mouse heart model to directly link reduced cytosolic Ca2+ buffering to increased atrial arrhythmogenesis. Taken together, we conclude that increasing cytosolic Ca2+ buffering may represent a novel therapeutic strategy to improve atrial contraction and reduce arrhythmogenesis in patients with AF.
METHODS
A detailed description of all methods is provided in the Supplemental Material.
Experimental protocols were approved by the ethics committees of Göttingen University (No. 10/9/15, 15/2/20 and No. 4/11/18) and conducted following the Declaration of Helsinki. Each patient gave written informed consent.
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Human Tissue Samples and Myocyte Isolation
Right atrial appendages were obtained from sinus rhythm patients (Ctrl) and patients in long-term persAF undergoing open-heart surgery (Tables S1 through S3). Excised right atrial appendages were either snap-frozen in liquid nitrogen for biochemical studies or subjected to a standard protocol3,18 for myocyte isolation. Right atrial myocytes were suspended in EGTA-free storage solution for subsequent simultaneous measurement of cellular electrophysiology and [Ca2+]i (Figure S1).
Cardiac Differentiation of Human iPSCs and Small Interfering RNA–Mediated cTnC Knockdown
Atrial iPSC-CMs were generated by subtype-directed differentiation of iPSCs from healthy donors, as previously described.19–21 In brief, directed feeder-free cardiac differentiation of iPSC lines was achieved through canonical WNT modulation with small molecules CHIR (day 0) and IWP2, followed by metabolic selection with lactate. For atrial subtype specification, 1 µmol/L retinoic acid (Sigma Aldrich) was added between day 3 and day 6. Between day 27 and day 30, purified iPSC-CMs were digested with TrypLE (Thermo Fisher Scientific) and sparsely plated on 1:60 Matrigel-coated borosilicate glass 10-mm No. 0 round coverslips at a density of 15 000 cells/cm2.
Human atrial iPSC-CMs in culture were transfected for 48 hours using predesigned small interfering RNAs (siRNAs) targeting the cardiac troponin C 1 gene (TNNC1; Thermo Fisher, s14273) or nonsilencing negative control siRNA (Thermo Fisher, 4390843) following provided manufacturer instructions. Transfected cells were used for subsequent downstream experiments.
Intracellular Calcium Measurement and Cellular Electrophysiology
Only rod-shaped myocytes with clear striations and defined margins, as observed in brightfield mode with the microscope ocular, were selected for measurements of [Ca2+]i and cellular electrophysiology. [Ca2+]i of right atrial myocytes was measured using the fluorescent Ca2+ indicator Fluo-3 AM, according to our previously published protocol.3 Simultaneously, the whole-cell ruptured patch-clamp technique was used to record membrane currents at 37 °C.3 Membrane currents were related to membrane capacitance and expressed in current density (pA/pF). Measurements of Ca2+ entry (integrated ICa,L) and SR Ca2+ content (integrated caffeine induced trainsient inward current) are expressed per liter total cell volume, which has been estimated based on a capacitance to volume relationship. Capacitance to volume relationship of atrial iPSC-CMs was estimated based on previously published data (4.57 pF/pL).22
Ca2+ sparks in Fluo-4 AM–loaded atrial iPSC-CMs were measured in separate experiments using a LSM 5 confocal microscopy system (Carl Zeiss, Jena, Germany) with a 40× oil objective in line-scan mode (512 pixels, 37.5 μm, 1302 Hz, 10 000 cycles, pinhole 67 µm) and Zen 2009 acquisition software. Field stimulation (2 Hz) was applied to myocytes for approximately 20 s, after which confocal line scans were performed during rest.
Optical action potentials (AP) were measured under field stimulation in 0.1× Fluovolt (Thermo Scientific)–loaded atrial iPSC-CMs in a bath solution containing (in mmol/L) CaCl2 2, glucose 10, HEPES 10, KCl 4, MgCl2 1, NaCl 140; pH=7.35 was adjusted with NaOH, on the heated (37 °C) stage of an epifluorescence microscope (λex=470 nm, λem=535 nm), optimized for high-speed photomultiplier signal capture.23,24
Determination of Atrial Cell Volume
A modification of the approach used by Walden et al25 was used to determine the capacitance to volume relationship in atrial myocytes, allowing Ca2+ fluxes and SR Ca2+ content to be expressed relative to total cell volume. Atrial myocytes from patients were loaded with the membrane-staining dye di-4-ANEPPS (2 μmol/L, 2 minutes) and imaged using serial z stacks (0.16 μm thickness, 63×1.2 NA water immersion objective, λex=488 nm, and λem=500–783 nm). Cell volume was calculated by multiplying the cross-sectional area with the z interval and assuming an accessible fraction of 0.65.
Skinned Fiber Preparation and Force Measurements
Skinned fibers were prepared as described previously.26 Resected muscle fibers were incubated for 24 hours in a 1% Triton X-100 solution for membrane permeabilization. After this skinning process, muscle strips were prepared (2–2.5 mm×0.3 mm).
For force measurements muscle fibers were installed in a force transducer system (Scientific Instruments, Heidelberg, Germany) and perfused with relaxation buffer containing (in mmol/L) imidazole 68, creatine phosphate 327, sodium azide 65, ethyleneglycol tetraacetic acid 380, MgCl2 203, dithioerythritol 154, ATP 605, and creatine kinase 400 U/mL. Free Ca2+ was increased stepwise according to Fabiato and Fabiato for measurement of contraction.27
Biochemical Studies
Expression of myofilament proteins was quantified by immunoblot, as previously described,3,28,29 and normalized to calsequestrin, which was unchanged in persAF compared with control samples (Tables S4 and S5).
Langendorff Experiments
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee in compliance with European Union Directive 2010/63/EU, and with the current version of the German Law on the Protection of Animals. Mouse hearts were perfused retrogradely on a Langendorff system, and 3 electrodes were placed on the epicardial surface to measure whole-heart and atrial electrophysiology. In addition, a stimulating electrode was used. To reduce the threshold for atrial arrhythmia induction, hearts were perfused with solution containing decreasing concentrations of potassium (5.4, 3.7, and 2.0 mmol/L). Diazoxide (300 µmol/L)30 was added to the perfusing solution (2.0 mmol/L potassium) as a final step. At each potassium concentration, various electrophysiological measurements were made, including the absolute refractory period and reaction to step burst pacing (400–4500 bpm) and shorter periods of high-frequency burst pacing (6000 bpm). Inducibility and duration of arrhythmic activity were measured and quantified.
Statistical Analysis
Normally distributed data (Shapiro-Wilk normality test) were compared using the unpaired 2-tailed Student t test. Differences between unpaired data with unequal variances were assessed using the Welch t test, which is indicated in the legends of all relevant figures. Nonnormally distributed data and all data sets with n<10 were compared with the Mann-Whitney U test. The Kruskal-Wallis test, followed by a Dunn post hoc test, was used to assess differences between 3 or more experimental groups. The simultaneous influence of 2 independent factors was appraised by using a 2-way ANOVA, followed by the Fisher Least Significant Difference post hoc test. Kaplan-Meier curves were compared using the Gehan-Breslow-Wilcoxon test. P<0.05 was considered statistically significant.
RESULTS
Reduced Ca2+ Buffering in Atrial Cardiac Myocytes From Patients With persAF
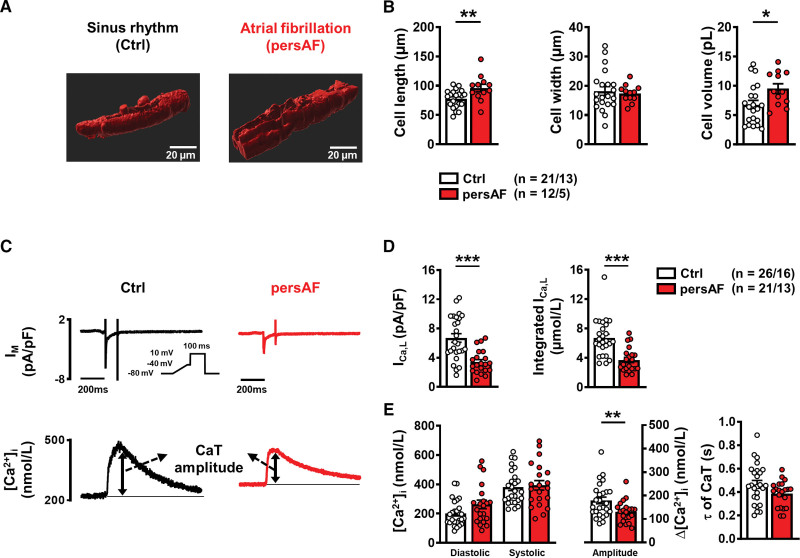
To quantify total cytosolic Ca2+ concentrations and cytosolic Ca2+ buffering, it is necessary to express sarcolemmal Ca2+ fluxes relative to total cell volume. Cell volume of atrial cardiac myocytes could be quantified from z-stack images, as described above (Figure 1A). The calculated volume was found to be higher in myocytes from patients with persAF, which appears to be mainly a result of cellular elongation, whereas cell width was not different (Figure 1B). Cell capacitance was measured at the beginning of electrophysiological experiments and was found to be comparable in both groups (persAF: 120.0±14.4 pF, n/N myocytes/patients=21/13 versus control: 100.5±9.4, n/N=26/16; P=0.25). We calculated the capacitance to volume ratio based on mean values of both parameters (persAF: 12.6±1.9 pF/pL, control: 14.9±2.2 pF/pL) and further analyzed the association between them, which we found to be strongly linear (Figure S2). These ratios were then applied to all individual capacitance measurements to estimate the volume of each individual cell undergoing electrophysiological measurement.
Figure 1.
Characterization of atrial myocytes isolated from patients without (Ctrl) and with persAF. A, Example 3-dimensional reconstruction of confocal z-stack images of atrial myocytes from Ctrl and persAF stained with di-4-ANEPPS. B, Mean±SEM cell dimensions and volume of control and persAF myocytes. C, ICa,L-triggered CaT in control and persAF atrial myocytes; representative simultaneous recordings of ICa,L (upper, inset, voltage-clamp protocol, 0.5 Hz) and triggered CaT (Fluo-3, lower). D, Mean±SEM peak ICa,L (left) and integrated ICa,L (right). E, Mean±SEM diastolic and systolic [Ca2+]i (left) and resulting CaT amplitude (middle), and time constant (τ) of decay (right). *P<0.05, **P<0.01, ***P<0.001 vs control. n/N=number of myocytes/patients. Normality of data was determined by Shapiro-Wilk test, whereas comparison was made using the Student t test with Welch correction and Mann-Whitney U test for normally and nonnormally distributed data, respectively. CaT indicates Ca2+ transient; Ctrl, control; and persAF, persistent atrial fibrillation.
Intracellular Ca2+ handling was investigated by simultaneous electrophysiological (whole-cell ruptured patch) and epifluorescence measurements (Figure 1C; Figure S3). A voltage-step protocol (0.5 Hz stimulation) was used to induce ICa,L, and in agreement with previous findings, both peak ICa,L amplitude and ICa,L integral were smaller in persAF versus Ctrl (Figure 1D). Consistent with previous findings,3 the diastolic Ca2+ levels tended to be higher in persAF, whereas amplitude of the ICa,L-triggered CaT was found to be smaller (Figure 1E).
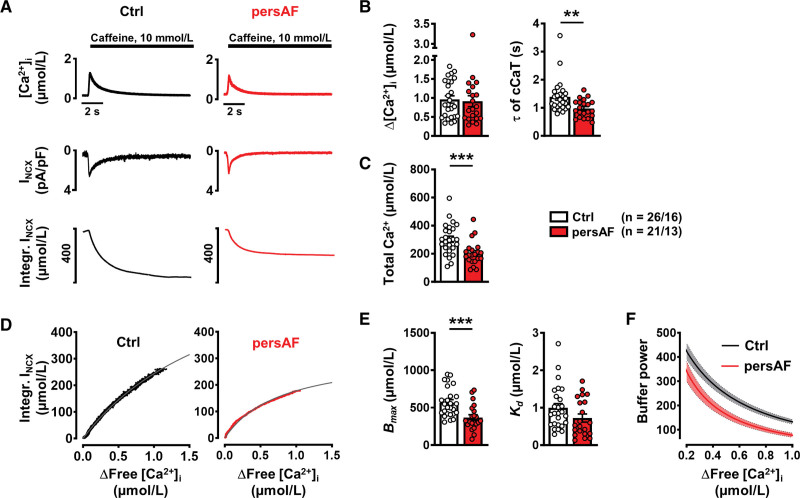
In subsequent experiments, SR Ca2+ content was quantified; myocytes were stimulated for 3 to 5 minutes using the same protocol, after which they were clamped at –80 mV, and 10 mmol/L caffeine was applied, causing complete SR Ca2+ release (Figure 2A). Interestingly, the amplitude of the caffeine-induced CaT (“free” Ca2+) was comparable in persAF versus control (Figure 2B). However “total” Ca2+, calculated from the integral of the resulting inward NCX current and normalized to cell volume, was found to be lower in persAF (Figure 2C). Not only does this finding indicate smaller SR Ca2+ content in persAF but, taken together with the comparable amplitude of the caffeine-induced CaT, it points towards altered Ca2+ buffering properties in persAF. To investigate this further, intracellular buffering was quantified by plotting total Ca2+ against cytosolic free Ca2+ during the caffeine-induced CaT, as shown in the representative traces of Figure 2D. The data were fitted with a Michaelis-Menten buffer curve:
Figure 2.
Caffeine-induced CaT and corresponding transient inward current (INCX) to assess SR Ca2+ content and buffering properties of atrial myocytes isolated from patients without (control) and with persAF. A, Representative caffeine-induced CaT (upper), associated INCX (middle) and integral of inward current, corrected for cell volume to give a measure of total Ca2+ (lower). B, Mean±SEM amplitude and time constant (τ) of decay of caffeine-induced CaT. C, Mean±SEM calculated total Ca2+. D, Representative buffer curves showing the relationship between cytosolic free Ca2+ and total Ca2+, fitted with a hyperbolic function. E, Mean±SEM maximum buffering capacity (Bmax, left) and dissociation constant (Kd, right), determined from buffer curves. F, Mean (line) ±SEM (shaded) of calculated individual total buffer power curves as a function of free [Ca2+]i. **P<0.01, ***P<0.001 vs control. n/N=number of myocytes/patients. Normality of data was determined by Shapiro-Wilk test and comparison was made using the Mann Whitney U test. Ctrl indicates control; and persAF, persistent atrial fibrillation.
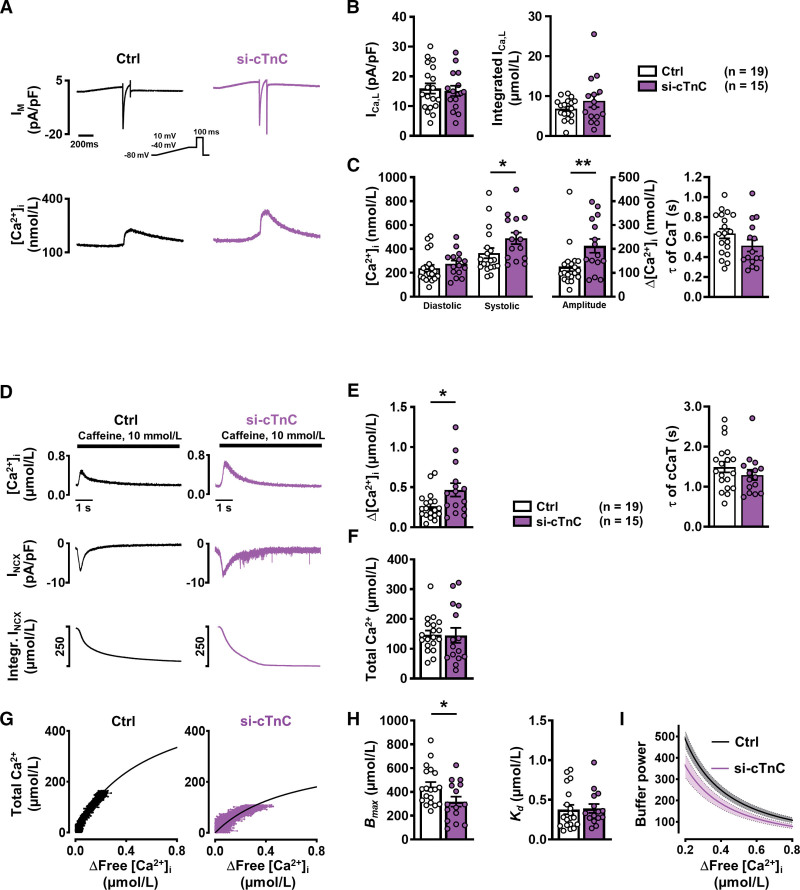
Maximum buffering capacity (Bmax) was found to be significantly lower in persAF versus control (Figure 2E), suggesting fewer cytosolic Ca2+ buffers, whereas the dissociation constant, Kd, was comparable in both groups. Figure 2F shows total buffer power (β) (see review by Smith and Eisner10), which is defined as the change of total Ca2+ divided by that of free Ca2+:
As previously described, total Ca2+ buffering represents the sum of the intrinsic Ca2+ buffering of the cardiac myocytes and the Ca2+ buffering provided by the added Fluo-3.31 However, Bmax remained lower in AF after correcting for the contribution of Fluo-3 to intracellular Ca2+ buffering (Figure S4).
Reduced Expression of cTnC Contributes to Impaired Ca2+ Buffering in persAF
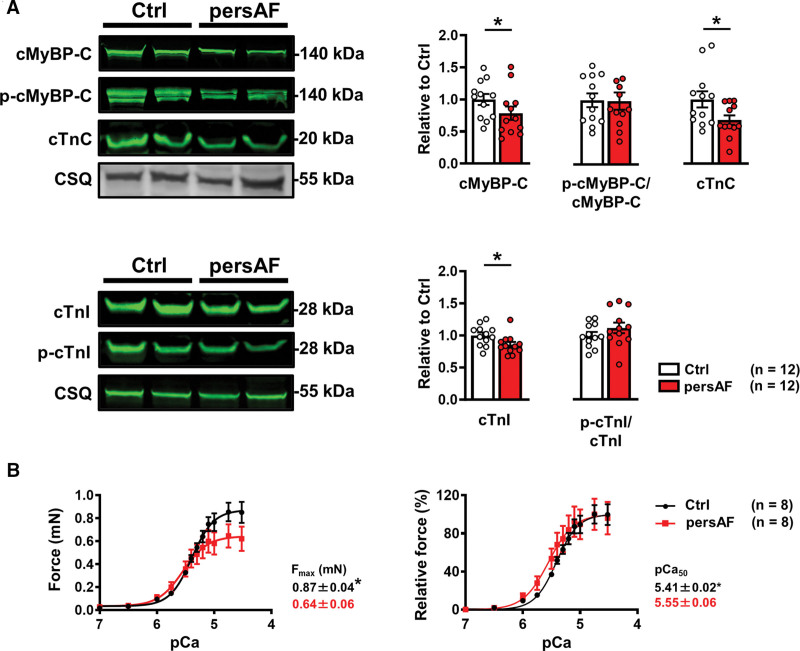
cTnC is a major cytosolic Ca2+ buffer; therefore, impairment of cTnC interaction with Ca2+ could alter the regulation of [Ca2+]i and cardiac contraction. The expression and phosphorylation of key myofilament proteins, which influence cytosolic Ca2+-myofilament interaction, were determined (Figure 3A; Figure S5; Figure S6). It is interesting that expression of the cardiac troponins cTnC and cTnI (cardiac troponin I) was lower in persAF, whereas phosphorylation of cTnI was comparable between groups. Because the cardiac troponins, particularly cTnC, mediate Ca2+-binding to myofilaments, lower troponin expression likely contributes to the smaller Bmax observed in persAF. The expression of cMyBP-C (cardiac myosin binding protein-C) was also lower in persAF versus Ctrl, with preserved phosphorylation levels (Figure 3A), which, together with reduced expression of cardiac troponins, points to a loss of myofilament proteins. Furthermore, expression of Myh6 (myosin heavy chain 6) and Tm1 (tropomyosin 1) were lower in persAF, whereas expression of Tm2 (tropomyosin 2) and α-actin were comparable with Ctrl (Figure S5). Skinned muscle fibers from persAF exhibited lower maximum force than control (Figure 3B), which may contribute to impaired fractional shortening of isolated cardiac myocytes from patients with persAF (Figure S3). Ca2+ sensitivity of force generation (pCa50) was higher in atrial muscle fibers from patients with persAF and may represent a compensatory mechanism for the reduced maximum force in persAF. Phosphorylation of MLC2a (atrial isoform of myosin light chain) and desmin was increased in persAF (Figure S6) and may contribute to increased myofilament Ca2+ sensitivity.17
Figure 3.
Myofilament protein expression and contractile response to cytosolic Ca2+ in control and persAF. A, Immunoblots (upper left) and quantification (upper right) of cMyBP-C (cardiac myosin binding protein-C), its phosphorylated state (P-cMyBP-C), and cTnC (cardiac troponin-C) in atrial samples from controls and patients with persAF, normalized to CSQ (calsequestrin), except for P-cMyBP-C, which was normalized to total cMyBP-C. Immunoblots (lower left) and quantification (lower right) of cTnI (cardiac troponin I) and its phosphorylated state (P-cTnI) in atrial samples from controls and patients with persAF, normalized to CSQ and total cTnI, respectively. B, Absolute (left) and normalized (right) force-pCa relationship of skinned muscle fibers of controls and patients with persAF with mean±SEM of maximum force (Fmax) and calcium sensitivity (pCa50). *P<0.05 vs control. n=number of patients. Normality of data was determined by Shapiro-Wilk test, whereas comparison was made using the Student t test with Welch correction. Ctrl indicates control; and persAF, persistent atrial fibrillation.
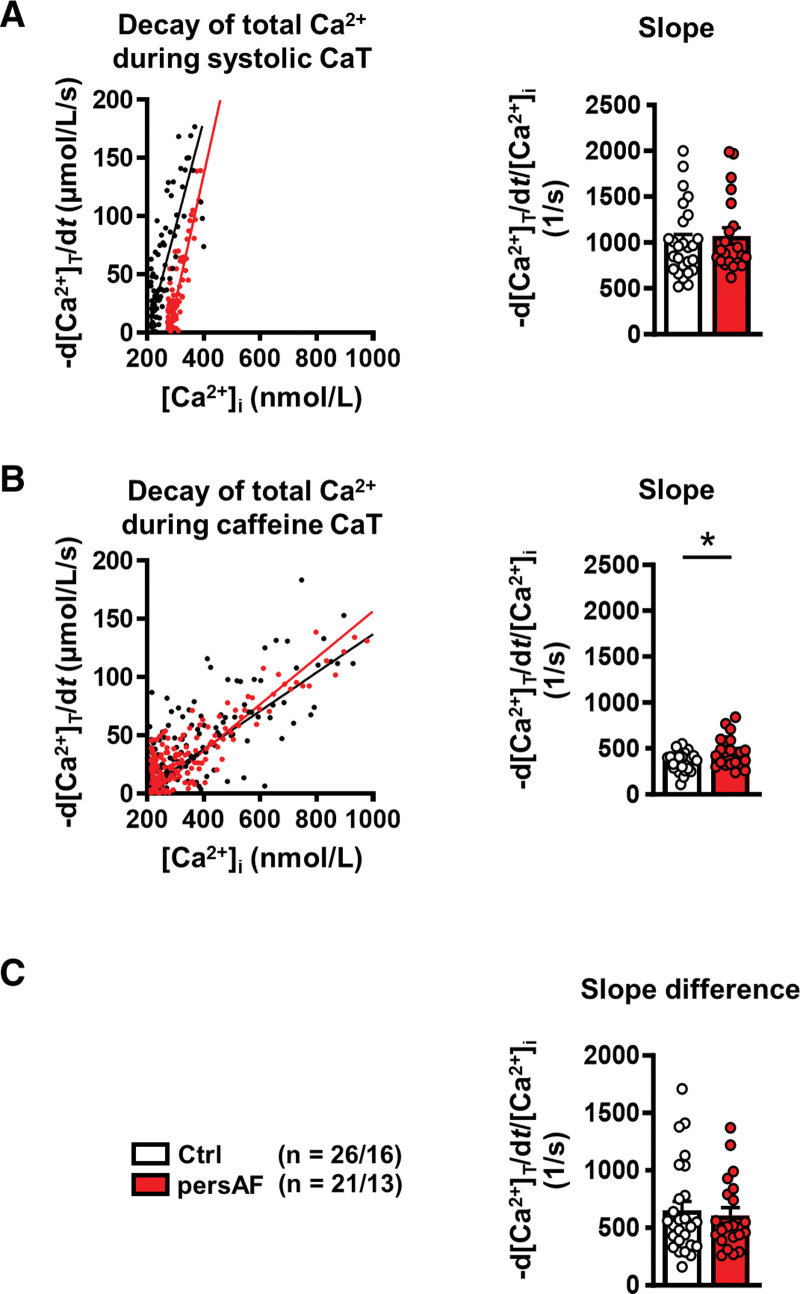
In addition to cTnC, SERCA is also an important buffer of cytosolic Ca2+ in cardiac myocytes.10 To quantify SERCA activity independent of cytosolic Ca2+ buffering, the difference in the decay of both the total caffeine-induced CaT and total systolic CaT (calculated from buffering properties) was analyzed, as previously described.23,32 Figure 4A shows example plots of the rate of decay of total Ca2+ against free Ca2+ concentration during the systolic CaT. The mean slope of this relationship was comparable in control and persAF. Decay of caffeine-induced CaT is SERCA-independent and results from sarcolemmal Ca2+ extrusion, mainly through NCX (Figure 2A). When plotting rate of decay of total Ca2+ against free Ca2+ concentration during the caffeine-induced CaT, the slope was higher in persAF, indicating faster removal of Ca2+ by NCX in persAF (Figure 4B). Contribution by SERCA could be ascertained by calculating the difference of the slope gradients in Figure 4A and 4B. It could be shown that SERCA contribution was comparable in control (60.7%±3.1%) and persAF (55.1%±2.6%; Figure 4C), indicating that altered buffering capacity in persAF is unlikely a result of changes in SERCA. This leaves the reduction in cTnC expression as an explanation for the reduced Ca2+ buffering capacity.
Figure 4.
Quantification of decay of total Ca2+ in atrial myocytes isolated from patients without (control) and with persAF. A, Representative rate of decay of total Ca2+ (-d[Ca2+]T/dt) plotted during systolic CaT against free [Ca2+]i (left) and slope of -d[Ca2+]T/dt plotted against [Ca2+]i (right). B, Representative rate of decay of total Ca2+ during caffeine-induced Ca2+ transient (-d[Ca2+]T/dt) plotted against the corresponding free [Ca2+]i (left) and slope of -d[Ca2+]T/dt during caffeine plotted against corresponding [Ca2+]i (right). C, Difference between slopes in A and B, indicating unaltered [Ca2+]i dependence of SERCA-mediated Ca2+ removal. *P<0.05 vs control. n/N=number of myocytes/patients. Normality of data was determined by Shapiro-Wilk test, whereas comparison was made using the Student t test with Welch correction and Mann-Whitney U test for normally and nonnormally distributed data, respectively. Ctrl indicates control; and persAF, persistent atrial fibrillation.
siRNA-Mediated cTnC Knockdown in Atrial iPSC-CM Phenocopies Ca2+ Buffering Characteristics of persAF
To further explore whether reduced cTnC is responsible for the altered buffering observed in persAF, an atrial iPSC-CM model with reduced cTnC protein expression was used. Atrial iPSC-CMs were differentiated as previously described and confirmed for atrial-specific markers (MLC2a and IK,ACh [acetylcholine activated inward rectifier K+ current]; Figure S7).19–21 cTnC knockdown was mediated by siRNA targeting cTnC (si-cTnC), and for the control group, atrial iPSC-CMs were treated with nonsilencing siRNA (Figure S8). For simultaneous epifluorescence and electrophysiological measurement, atrial iPSC-CMs were loaded with Fluo-3 AM and stimulated by voltage-clamp control in the whole cell ruptured patch configuration (Figure 5A). ICa,L was induced by a voltage-step protocol (0.5 Hz stimulation), and peak current and current integral were found to be comparable in control and si-cTnC (Figure 5B), whereas the amplitude of the ICa,L-triggered CaT was larger in si-cTnC versus control (Figure 5C). iPSC-CMs were subsequently clamped at –80 mV, and 10 mmol/L caffeine was applied, causing total release of SR Ca2+ (Figure 5D). The amplitude of the caffeine-induced CaT was larger in si-cTnC; however, when the integral of the resulting inward NCX current was quantified and total Ca2+ was calculated, these parameters were found to be similar in si-cTnC and control, pointing to comparable SR Ca2+ load (Figure 5D through 5F). Intracellular buffering was quantified, as described previously, by plotting total Ca2+ against cytosolic free Ca2+ during the caffeine-induced CaT and fitting the data with a hyperbolic curve (Figure 5G). Analysis revealed that Bmax was smaller in si-cTnC versus control, whereas Kd was comparable between both groups (Figure 5H; Figure S9), thus mimicking the Ca2+ buffering characteristics of persAF. In addition, buffer power was lower in si-cTnC compared with control (Figure 5I). Contribution of NCX and SERCA to cytosolic Ca2+ removal remained unaltered in si-cTnC iPSC-CMs (Figure S10).
Figure 5.
Ca2+ handling and Ca2+ buffering properties in atrial iPSC-CMs with normal (control) and reduced (si-cTnC) cTnC levels. A, Representative simultaneous recordings of ICa,L (upper, inset, voltage-clamp protocol, 1 Hz) and triggered CaT (lower). B, Mean±SEM peak ICa,L (left) and integrated ICa,L (right) in control (siRNA ns) and si-cTnC (siRNA cTnC) iPSC-CMs. C, Mean±SEM diastolic and systolic [Ca2+]i (left) and resulting CaT amplitude (middle), and time constant (τ) of decay (right). D, Representative caffeine-induced CaT (upper), associated INCX (middle) and integral of inward current, corrected for cell volume to give a measure of total Ca2+ (lower). E, Mean±SEM amplitude (left) and time constant (τ) of decay (right) of caffeine-induced CaT. F, Mean±SEM calculated total Ca2+. G, Buffer curves showing the relationship between cytosolic free Ca2+ and total Ca2+, fitted with a hyperbolic function. H, Mean±SEM maximum buffering capacity (Bmax, left) and dissociation constant (Kd, right), determined from buffer curves. I, Mean±SEM of calculated individual total buffer power curves as a function of free [Ca2+]i. *P<0.05, **P<0.01 vs control. n=number of myocytes (2–4 differentiations). Normality of data was determined by Shapiro-Wilk test, whereas comparison was made using the Student t test and Mann-Whitney U test for normally and nonnormally distributed data, respectively. Ctrl indicates control; cTnC, cardiac troponin C; CaT, Ca2+ transient; iPSC-CM, induced pluripotent stem cell–derived cardiac myocyte; ns, nonsilencing; and siRNA, small interfering RNA.
Potential Proarrhythmic Consequences of Reduced cTnC Levels
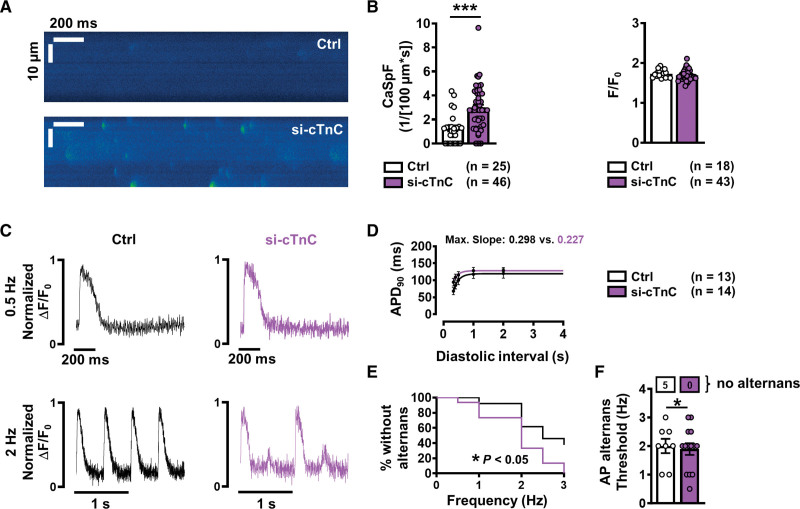
It has previously been shown that increased Ca2+ leak from the SR provides a basis for arrhythmogenesis in persAF.3,7,8 To investigate whether reduced cytosolic Ca2+ buffering, specifically in the form of reduced cTnC expression, could play a proarrhythmic role, diastolic SR Ca2+ release was measured and quantified in atrial si-cTnC versus control (siRNA nonsilencing) iPSC-CMs. To this end, confocal line scans were performed on atrial iPSC-CMs during rest, after a brief period (20 s) of field stimulation (2 Hz). Line scan analysis revealed significantly higher Ca2+ spark frequency in si-cTnC compared with control (Figure 6A and 6B), indicating increased SR Ca2+ leak. In addition, we found increased SR Ca2+ leak using the previously described tetracaine protocol33 (Figure S11). Moreover, the leak-load relationship was shifted leftward in persAF, indicating increased SR Ca2+ leak at any given SR Ca2+ content. Immunoblot analysis revealed comparable expression levels of RyR2, CaMKII, and junctophilin-2, as well as phosphorylated RyR2 and CaMKII, in si-cTnC compared with control iPSC-CMs (Figure S12).
Figure 6.
Incidence of Ca2+ sparks and action potential (AP) alternans in atrial iPSC-CMs with normal (control) and reduced (si-cTnC) cTnC levels. A, Representative confocal line scans showing SR Ca2+ release in the form of Ca2+ sparks in control (siRNA ns) and si-cTnC (siRNA cTnC) iPSC-CMs. B, Mean±SEM Ca2+ spark frequency (CaSpF, left) and amplitude (right). C, Representative normalized traces of AP at 0.5 Hz (upper) and 2 Hz (lower) in control (left) and si-cTnC iPSC-CMs. D, AP duration at 90% repolarization (APD90) at increasing diastolic intervals (AP restitution), fitted with a 1-phase association nonlinear function to determine maximum curve slope. E, Kaplan-Meier plot indicating the percentage of iPSC-CMs without alternans in relation to the respective pacing frequency. F, Mean±SEM alternans threshold frequency. Number of myocytes without AP alternans are shown in boxes above. ***P<0.001, *P<0.05 vs control. n=number of myocytes (2 or 3 differentiations). Comparison was made using the unpaired Student t test, the Mann-Whitney U test, and the Gehan-Breslow-Wilcoxon test (E). Ctrl indicates control; cTnC, cardiac troponin C; iPSC-CM, induced pluripotent stem cell–derived cardiac myocyte; ns, nonsilencing; and siRNA, small interfering RNA.
In accordance with our in vitro experiments, computational modeling revealed that reduction of cytosolic Ca2+ buffering increases incidence of Ca2+ waves and delayed after depolarizations (Figure S13).4,34,35
Optical AP measurements revealed no difference in AP duration or restitution between control and si-cTnC iPSC-CMs (Figure 6C and 6D). However, the incidence of AP alternans at lower pacing frequencies was significantly larger in cells with reduced cTnC (Figure 6E and 6F). The maximum slope of the restitution curve did not exceed 1 in either group, indicating that voltage alternans of the AP is driven by intracellular Ca2+ aberrations.
Taken together, these data demonstrate that cTnC reduction is sufficient to reproduce the phenotype of impaired Ca2+ buffering observed in persAF, including increased SR Ca2+ leak and alternans, both of which are strongly arrhythmogenic.3,7,8
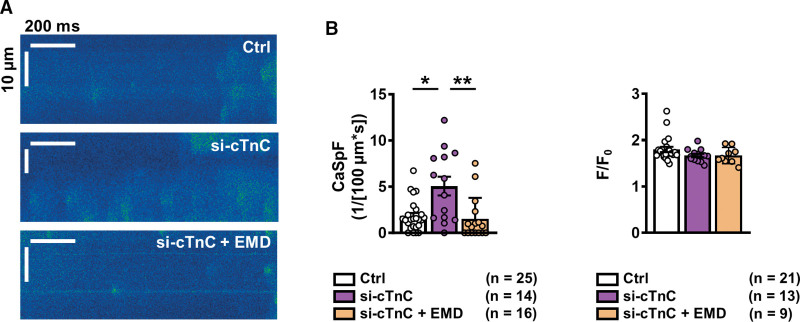
To ascertain whether improving Ca2+ buffering can ameliorate increased SR Ca2+ leak in si-cTnC iPSC-CMs, si-cTnC iPSC-CMs were pretreated with the Ca2+ sensitizer EMD57033 (5 µmol/L, pretreatment for 5 minutes),23 and confocal line scan analysis was repeated. Although the amplitude of Ca2+ sparks was similar between treated and nontreated groups, Ca2+ spark frequency in si-cTnC iPSC-CMs was significantly reduced by EMD57033 (Figure 7A and 7B). Furthermore, Ca2+ spark frequency in the EMD57033-treated group was similar to that in control iPSC-CMs, thus confirming that increasing buffering can normalize SR Ca2+ leak.
Figure 7.
Effect of Ca2+ sensitization on Ca2+ sparks in atrial iPSC-CMs with reduced (si-cTnC) cTnC levels. A, Representative confocal line scans of atrial iPSC-CMs with normal (control, siRNA ns) and reduced (si-cTnC, siRNA cTnC) cTnC levels, pretreated with EMD57033 (EMD, 5 µmol/L). B, Mean±SEM Ca2+ spark frequency (CaSpF, left) and amplitude (right). *P<0.05, **P<0.01 vs control and si-cTnC. n=number of myocytes (2 differentiations). Comparison was made using the Kruskal-Wallis test followed by the Dunn post hoc test. Ctrl indicates control; cTnC, cardiac troponin C; iPSC-CM, induced pluripotent stem cell–derived cardiac myocyte; ns, nonsilencing; and siRNA, small interfering RNA.
Desensitization of Myofilaments to Ca2+ Causes Atrial Arrhythmia
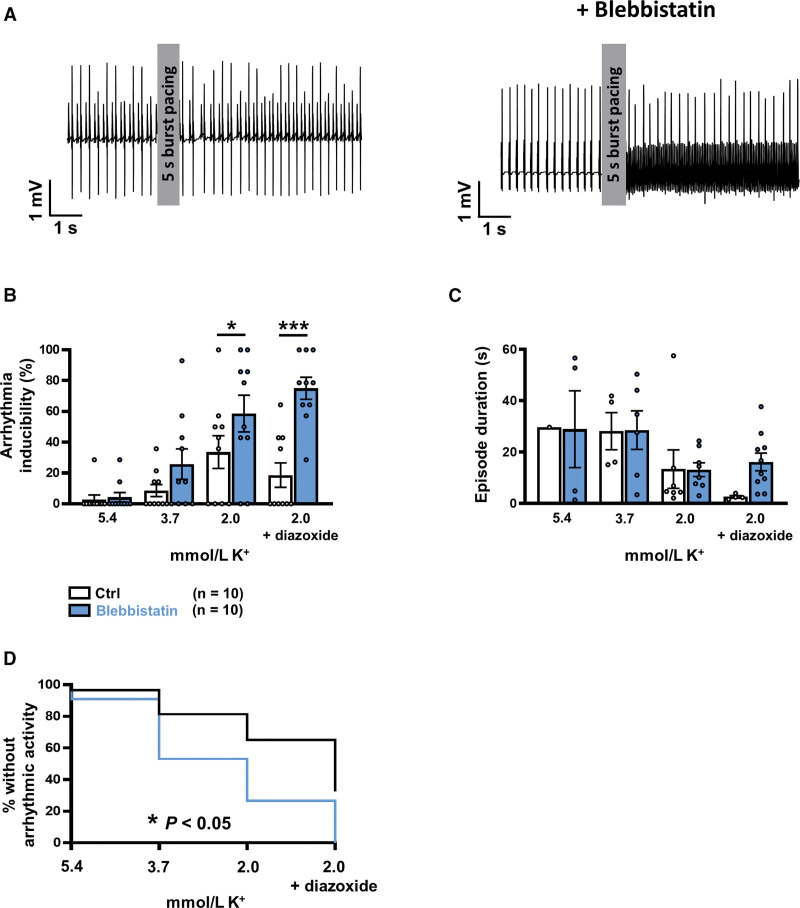
To determine whether reduced cytosolic Ca2+ buffering by myofilaments alone can induce arrhythmic activity in the atria, blebbistatin was applied to Langendorff-perfused mouse hearts to reduce myofilament Ca2+ sensitivity, during which atrial electrograms were recorded (Figure S14). Blebbistatin significantly increased the inducibility of atrial arrhythmic activity after burst-pacing (potassium in perfusing solution: 2 mmol/L, both with and without diazoxide, Figure 8A and 8B). However, the duration of atrial arrhythmic episodes was comparable even in the presence of blebbistatin (Figure 8C). Figure 8D shows a Kaplan-Meier curve of the percentage of hearts without atrial arrhythmic activity after burst pacing plotted against decreasing potassium concentrations. Blebbistatin significantly altered this curve, pointing to a higher susceptibility to atrial arrhythmic activity.
Figure 8.
Langendorff experiments in mouse heart. A, Representative atrial electrogram traces showing effect of burst pacing (100 Hz) in the absence (control, left) and presence (right) of blebbistatin (5 µmol/L) (2 mmol/L K+ in both). B, Grouped bar chart showing mean±SEM inducibility of atrial arrhythmic activity for each potassium level, differentiated by the presence or absence of blebbistatin. The chart highlights significant main effects of blebbistatin, F(1, 72)=20.06, P<0.0001, and potassium, F(3, 72)=14.91, P<0.0001. C, Mean±SEM arrhythmic episode duration for each potassium level, in the presence or absence of blebbistatin. The graph highlights the significant effect of potassium, F(3, 36)=3.52, P<0.05. D, Kaplan-Meier plot showing the percentage of hearts without arrhythmic activity. *P<0.05, ***P<0.001 vs control. n=number of hearts. Comparison using 2-way ANOVA followed by a Fisher Least Significant Difference post hoc test (B and C) and the Gehan-Breslow-Wilcoxon test (D). Ctrl indicates control.
DISCUSSION
In the present study, we observed impaired cytosolic Ca2+ buffering in atrial myocytes from patients with persAF and analyzed the underlying molecular substrate and its contribution to atrial arrhythmogenesis. Analysis of transmembrane Ca2+ fluxes during systolic and caffeine-induced CaT enabled estimation of total Ca2+ in relation to free cytosolic Ca2+. Our experiments revealed reduced total Ca2+ buffering capacity in persAF, likely because of degradation of myofilament proteins, which represent a major Ca2+ buffer in cardiac myocytes. Myofilaments play an important role in cytosolic Ca2+ handling, and therefore, altered myofilament expression may have direct consequences on cytosolic Ca2+ homeostasis. Here we demonstrate, for the first time, that reduction of cTnC expression decreases intracellular Ca2+ buffering and increases the incidence of both SCaEs and AP alternans in atrial iPSC-CMs, thereby phenocopying the arrhythmogenic Ca2+ handling phenotype observed in atrial myocytes from patients with persAF.3,7,8
Reduced Ca2+ buffering leads to a higher change in free cytosolic Ca2+ per total Ca2+ released from the SR and therefore amplifies consequences of higher incidence of SCaEs as a mechanism of increased ectopic activity in persAF. Last, reducing cytosolic Ca2+ buffering in an in vitro mouse model increased susceptibility to pacing-induced atrial arrhythmia, validating the direct mechanistic link between impaired cytosolic Ca2+ buffering and atrial arrhythmogenesis in clinical persAF.
Impaired Contractile Function in persAF
It has been established that persAF is associated with Ca2+ handling abnormalities that contribute to impaired contractility and arrhythmogenesis; reduced ICa,L has been widely shown to be a characteristic hallmark of AF-associated remodeling and a major contributor to AP shortening in persAF, promoting maintenance of reentry.3,36–38 In addition, reduced ICa,L triggers smaller Ca2+ release from the SR, thereby contributing to impaired contractile function of atrial myocytes from persAF.
Impaired contractility is a major hallmark of AF-associated remodeling.15,39,40 During an AF episode, contractile function of the atria is mainly constrained because of the fast and uncoordinated atrial excitation. However, the impaired contractile function persists for several weeks even after the cardioversion of AF back to sinus rhythm, leading to a high risk of atrial thromboembolism and stroke, despite sinus rhythm maintenance.41 Similarly, force of contraction is clearly reduced in atrial preparations from patients with AF when stimulated in vitro with a constant frequency.40 Impaired Ca2+ handling40,42 and structural remodeling, including increased fibrosis, have been suggested to contribute to AF-associated contractile dysfunction.2 Other studies of AF have shown that reduced contractility even persists in myofibril preparations lacking sarcolemma and Ca2+ handling machinery,16,17 demonstrating that reduction in myofibrillar maximal active tension in persAF must be attributable to defects in the myofilaments themselves. Furthermore, and in accordance with our results, it has been shown that loss of myofilament proteins is a major mechanism contributing to impaired atrial contractility.14,15,17,39,40,43,44 Meanwhile, mechanistic studies have revealed that degradation of cardiac troponins mainly results from increased activation of the Ca2+-dependent protease calpain during high atrial stimulation frequencies,14,43,45 suggesting that calpain inhibition may represent a future therapeutic target in preventing AF-associated contractile remodeling.
In contrast with impaired maximal force development associated with AF, our present investigation, in accordance with a previous study,17 demonstrated higher myofilament Ca2+ sensitivity in atrial preparations from patients with persAF. Increased phosphorylation of MLC2a is likely a major contributor to the increased Ca2+ sensitivity.46,47 However, results on phosphorylation of MyBP-C (thought to increase myofilament Ca2+ sensitivity) are controversial, showing increased,17 decreased,48 or unaltered (present study) phosphorylation levels.
Determinants of Increased Incidence of SCaEs in persAF
Increased incidence of SCaEs originating from the SR during diastole is a well-accepted mechanism underlying enhanced ectopic activity, triggering AF episodes and contributing to AF progression and maintenance.3,5,8,9 Ca2+ removal from the cytosol by NCX brings 3 Na+ ions into the cell per extruded Ca2+ ion. This is an electrogenic process, leading to membrane depolarizations (delayed afterdepolarizations) which, if large enough, could trigger a new AP, resulting in ectopic activity. Several mechanisms have been identified to amplify consequences of leaky RyR2 channels: (1) higher SR Ca2+ load, for example as a result of increased excitation frequencies in AF, escalates SR Ca2+ leak, because of the exponential increase of leak-load relationship49; (2) expression and activity of NCX are increased in persAF, resulting in increased arrhythmogenic transient inward current in response to a given diastolic Ca2+ release from the SR3; (3) the distance between RyR clusters is reduced in AF, thereby enhancing the propagation of Ca2+ waves50,51; (4) impaired buffering of cytosolic Ca2+ promotes occurrence of atrial SCaEs in persAF, as demonstrated in this study.
Atrial Arrhythmias and Ca2+ Buffering
It has been established that alterations in cytosolic Ca2+ buffering contribute to ventricular arrhythmias.13,23,52 To the best of our knowledge, there are currently no studies investigating the role of reduced Ca2+ buffering in atrial arrhythmogenesis in humans. It is often overlooked that only 1% of cytosolic Ca2+ is free and detected by conventional Ca2+ indicators such as Fluo-3, whereas the remainder is bound to Ca2+ buffers.10 Because the myofilament protein cTnC is one of the major cytosolic Ca2+ buffers in cardiac myocytes,10 it can be assumed that even minor changes in Ca2+ binding to cTnC can have major effects on free cytosolic Ca2+, which could play an important role in cardiac arrhythmias. Early computational modeling studies, for example, suggested that Ca2+ buffers critically limit the diffusion of locally released Ca2+ and hamper activation of neighboring Ca2+ release sites, thereby preventing the occurrence of arrhythmogenic Ca2+ waves.53 Accordingly, experimental reduction of cytosolic Ca2+ buffering caused increased Ca2+ sparks and intracellular propagation of arrhythmogenic Ca2+ waves.54 Here we provide evidence that genetic downregulation of cTnC leads to significant reduction of cytosolic Ca2+ buffering and consecutively increased Ca2+ spark frequency. Furthermore, Ca2+ spark frequency could be reduced by pharmacological treatment with a Ca2+ sensitizing agent, EMD57033 (Figure 7). It is important to note that in si-cTnC iPSC-CMs, we found no difference, compared with control, in expression levels of RyR2, CaMKII, and junctophilin-2, nor the phosphorylated levels of RyR2 and CaMKII. Although RyR2 hyperphosphorylation is a well-accepted mechanism underlying increased diastolic Ca2+ leak in AF, the unaltered expression levels we found in our si-cTnC iPSC-CM model allowed us to focus on the contribution of reduced buffering to altered Ca2+ handling and arrhythmogenic activity.
Although several previous studies have investigated Ca2+ buffering properties in various animal models of AF, there have been contradictory results. Greiser et al found increased cytosolic Ca2+ buffering in a rabbit model after 5 days of atrial pacing.11 They hypothesized that their observation of reduced cTnI phosphorylation, causing increased Ca2+-cTnC binding, prevents detrimental effects of Ca2+ overload induced by rapid pacing. One could speculate that this may be an early response to high atrial stimulation frequencies, whereas loss of myofilament proteins, including cTnC, may represent a hallmark of late-stage remodeling, as shown by others.14,50,55 Interestingly, mavacamten, a recently approved compound for hypertrophic cardiomyopathy treatment, reduces myofilament Ca2+ affinity and was found to increase incidence of AF in the PIONEER-HCM study (Phase 2 Open-label Pilot Study Evaluating Mavacamten in Subjects With Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction),56,57 suggesting that reduced Ca2+ buffering may indeed facilitate occurrence of atrial arrhythmias.
We observed AP alternans at low pacing frequencies in atrial iPSC-CMs with reduced Ca2+ buffering because of cTnC knockdown. Primary AP alternans is assumed to arise during pathological (steep) AP restitution, which was not observed here, pointing to underlying Ca2+-driven alternans, which can canonically arise because of slow removal of Ca2+ into the SR, or in conditions of increased RyR2-mediated Ca2+ release into the cytosol. Because we detected significantly increased incidence of diastolic Ca2+ leak in atrial iPSC-CMs with reduced cTnC, we suggest that alternans arises at lower pacing rates, predominantly because of increased SCaEs, secondary to reduced buffer availability. This is in line with a previous modeling study suggesting that reduced cTnC-related Ca2+ buffering can increase Ca2+ alternans and also AP alternans.58
In addition to demonstrating the cellular arrhythmogenic phenotype caused by cTnC downregulation, we established the first ex vivo mouse heart model to investigate effects of reduced buffering on atrial arrhythmogenesis; we provided evidence at the whole-organ level that myofilament desensitization to Ca2+ increases susceptibility to atrial arrhythmia. Therefore, we hypothesize that reduced cytosolic Ca2+ buffering may play an important role in the progression and maintenance of AF in patients with persAF.
Potential Limitations
In our study, we collected samples from only 1 atrial region (right atrial appendage). Our findings may therefore not apply fully to other regions of the atria. Expression of α-actin and Tm2, for example, which was unaltered in our study of right atrial appendages, has been shown to be increased and decreased in left atrial biopsies from patients with AF, respectively.44 The individuals from whom we obtained tissue samples included only patients who underwent coronary bypass or valve replacement surgery. Such individuals have numerous comorbidities, and the phenotype of atrial cardiac myocytes from our Ctrl patients may be different from nondiseased controls. Furthermore, it is unclear whether the mechanisms identified here also apply to patients with persAF without any heart disease. However, impaired contractile function is a common clinical finding throughout all atrial regions and in all patients with persAF.2,40 Furthermore, ectopic activity occurs in right and left atria from patients with persAF, suggesting that increased incidence of SCaEs occurs throughout the whole atria. Here we demonstrate for the first time a direct mechanistic link between atrial hypocontractility and ectopic activity. Future clinical studies will be required to reveal whether there is a direct correlation between local hypocontractility and occurrence of ectopic activity in the different regions of the atria.
Our data suggest that loss of myofilament proteins and reduced expression of the major Ca2+ buffer cTnC are important contributors to reduced Ca2+ buffering in atrial cardiac myocytes from patients with persAF. There are numerous additional buffers that may contribute to impaired cytosolic Ca2+ buffering in AF, including the giant sarcomere protein titin,59 as well as the important buffers SERCA and the sarcolemma.10 However, activity of SERCA was comparable in persAF versus control cardiac myocytes investigated in our cohort (Figure 4), and cell capacitance as a marker for total membrane area was also comparable, making altered Ca2+ binding to SERCA and sarcolemma unlikely. Furthermore, the contribution of mitochondrial Ca2+ uptake to cytosolic Ca2+ buffering is controversial.60–63 However, several studies have observed no impact of mitochondrial Ca2+ uptake on the amplitude of cytosolic Ca2+ transients, even during β-adrenergic stimulation where relevant Ca2+ accumulation into the mitochondrial matrix (to stimulate Krebs cycle dehydrogenases) occurs.64–66 These observations render a significant contribution of mitochondria to cytosolic Ca2+ buffering unlikely. It needs to be considered that the concentration of cytosolic Ca2+ buffers is 3 times higher in the atria than in the ventricles.10 This may also explain the relatively high buffer power observed in the present study.10
Because neither genetic mouse models of impaired myofilament Ca2+ binding nor specific pharmacological Ca2+ desensitizing agents are available, in the present study, we used the myosin ATPase inhibitor blebbistatin to indirectly modulate myofilament Ca2+ sensitivity in Langendorff-perfused mouse hearts. Blebbistatin is widely used as a contractile uncoupling agent for electrophysiological studies and has been shown to reduce Ca2+ sensitivity (pCa50) of mouse skinned fiber preparations in a concentration-dependent manner.52 Although minimal or nonsignificant effects on cardiac ion channels and APs by blebbistatin have been reported,67 unspecific effects on ion channels cannot be completely excluded, in particular under the experimental conditions used in our model. However, atrial refractory periods, recorded at each experimental step (Figure S14B), did not differ between blebbistatin-treated and untreated hearts across the various K+ concentrations. This suggests that increased susceptibility to atrial arrhythmia after blebbistatin treatment is indeed a result of impaired cytosolic Ca2+ buffering. Nevertheless, mouse models in general do not phenocopy all important aspects of human cardiac electrophysiology. Further extensive work in large-animal AF models will be required to investigate the time course and pathophysiological role of impaired Ca2+ buffering under conditions similar to those in patients who develop persAF.
Conclusions and Potential Significance
Here we provide evidence for a direct link between impaired atrial contractility in persAF and arrhythmogenesis. Our data show that loss of myofilament proteins, including reduced expression of cTnC, leads to reduced cytosolic Ca2+ buffering, which promotes the occurrence of SCaEs and increases susceptibility to atrial arrhythmia.
Because loss of myofilament proteins represents a rather late phenomenon in AF remodeling, this mechanism is likely to contribute to the chronification of the arrhythmia. Furthermore, because free cytosolic Ca2+ increase is believed to play a major role in electrical and structural remodeling,45,68,69 reduced cytosolic Ca2+ buffering could therefore intensify remodeling in response to atrial tachycardia. In summary, our data suggest that, although the arrhythmogenic mechanisms of impaired Ca2+ buffering are not yet fully understood, the development of new strategies, specifically targeting intracellular Ca2+ buffering, may open novel therapeutic avenues to prevent progression and maintenance of AF. Already existing Ca2+ sensitizers, such as levosimendan and the indirectly acting omecamtiv mecarbil, may represent promising lead compounds.70,71 Furthermore, nutrient supplements such as β-alanine and taurine, which have been demonstrated to increase intracellular Ca2+ buffering in cardiac myocytes, may be considered as valuable additions to currently available therapeutics used in AF management.72,73
ARTICLE INFORMATION
Acknowledgments
The authors are grateful to Lucie Carrier (Hamburg, Germany) for providing the antibody against phosphorylated cMyBP-C, and they would also like to thank David A. Eisner (Manchester, United Kingdom) for helpful discussion. Furthermore, the authors thank Ines Müller and Brigitte Korff for technical assistance and Maren Dilaj for secretarial help.
Sources of Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft to N.V. (DFG, VO 1568/3-1, VO1568/4-1) and to N.V. and S.E.L. (IRTG1816, SFB1002 and under Germany’s Excellence Strategy-EXC 2067/1-390729940), from the Else-Kröner-Fresenius Foundation to N.V. (EKFS, 2016_A20), from the German Center for Cardiovascular Research to N.V. (DZHK, 81X4300102, 81X4300115, 81X4300112) and to A.E. (D81X4300123), and from the Bundesministerium für Bildung und Forschung (BMBF) subproject of the German Network for RASopathy Research (GeNeRARe) to G.K. (01GM1902D). This work was further supported by scholarships from the Göttingen Promotionskolleg für Medizinstudierende, funded by the Jacob-Henle-Programm and the Else-Kröner-Fresenius-Stiftung to V.M. and from the German Academic Exchange Service to I.S. and N.I. We thank the Clinic for Cardiology and Pneumology at the University Medical Center Göttingen and the Deutsche Stiftung für Herzforschung (F/13/20) for funding provided to A.E.
Disclosures
None.
Supplemental Material
Supplemental Methods
Tables S1–S5
Figures S1–S14
Reference 74
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- AP
- action potential
- Bmax
- maximum buffering capacity
- CaT
- Ca2+ transient
- cMyBP-C
- cardiac myosin binding protein-C
- Ctrl
- sinus rhythm control
- cTnC
- cardiac troponin C
- cTnI
- cardiac troponin I
- ICa,L
- L-type Ca2+ current
- iPSC-CM
- induced pluripotent stem cell-derived cardiac myocyte
- Kd
- equillibrium dissociation constant
- Myh6
- myosin heavy chain 6
- MLC2a
- myosin light chain 2a (atrial isoform)
- NCX
- Na+-Ca2+ exchanger
- persAF
- persistent atrial fibrillation
- RyR2
- cardiac ryanodine receptor type 2
- SCaEs
- spontaneous Ca2+ release events
- SERCA
- sarcoplasmic reticulum Ca2+-ATPase
- si-cTnC
- small interfering RNA targeting cardiac troponin C
- siRNA
- small interfering RNA
- SR
- sarcoplasmic reticulum
- Tm1
- tropomyosin 1
- Tm2
- tropomyosin 2
F.E. Fakuade, D. Hubricht, and V. Möller contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.066577.
For Sources of Funding and Disclosures, see pages 557–558.
Circulation is available at www.ahajournals.org/journal/circ .
Contributor Information
Funsho E. Fakuade, Email: funsho.fakuade@med.uni-goettingen.de.
Izzatullo Sobitov, Email: izzatullo.sobitov@stud.uni-goettingen.de.
Aiste Liutkute, Email: aiste.liutkute@med.uni-goettingen.de.
Yannic Döring, Email: yannic.doering@med.uni-goettingen.de.
Fitzwilliam Seibertz, Email: will.seibertz@med.uni-goettingen.de.
Fereshteh Haghighi, Email: fereshteh.haghighi@med.uni-goettingen.de.
Sören Brandenburg, Email: soeren.brandenburg@med.uni-goettingen.de.
Khaled Alhussini, Email: alhussini_k@ukw.de.
Nadezda Ignatyeva, Email: nadezda.ignatyeva@med.uni-goettingen.de.
Aschraf El-Essawi, Email: aelessawi@aol.com.
Ahmad Fawad Jebran, Email: fawad.jebran@med.uni-goettingen.de.
Marius Großmann, Email: marius.grossmann@med.uni-goettingen.de.
Bernhard C. Danner, Email: bernd.danner@med.uni-goettingen.de.
Hassina Baraki, Email: hassina.baraki@med.uni-goettingen.de.
Constanze Schmidt, Email: constanze.bening@googlemail.com.
Samuel Sossalla, Email: Samuel.Sossalla@innere.med.uni-giessen.de.
Ingo Kutschka, Email: ingo.kutschka@med.uni-goettingen.de.
Constanze Bening, Email: constanze.bening@googlemail.com.
Christoph Maack, Email: Maack_C@ukw.de.
Wolfgang A. Linke, Email: wlinke@uni-muenster.de.
Jordi Heijman, Email: jordi.heijman@medunigraz.at.
George Kensah, Email: george.kensah@med.uni-goettingen.de.
Antje Ebert, Email: antje.ebert@med.uni-goettingen.de.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. doi: 10.1016/j.hrthm.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J Cardiovasc Pharmacol. 2008;52:293–299. doi: 10.1097/FJC.0b013e318171924d [DOI] [PubMed] [Google Scholar]
- 6.Dobrev D, Voigt N, Wehrens XHT. The ryanodine receptor channel as a molecular motif in atrial fibrillation: pathophysiological and therapeutic implications. Cardiovasc Res. 2011;89:734–743. doi: 10.1093/cvr/cvq324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schöndube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836 [DOI] [PubMed] [Google Scholar]
- 8.Hove-Madsen L, Llach A, Bayes-Genís A, Roura S, Font ER, Arís A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. [DOI] [PubMed] [Google Scholar]
- 9.Bode D, Pronto JRD, Schiattarella GG, Voigt N. Metabolic remodelling in atrial fibrillation: manifestations, mechanisms and clinical implications [published online May 30, 2024]. Nat Rev Cardiol. doi: 10.1038/s41569-024-01038-6 [DOI] [PubMed] [Google Scholar]
- 10.Smith GL, Eisner DA. Calcium buffering in the heart in health and disease. Circulation. 2019;139:2358–2371. doi: 10.1161/CIRCULATIONAHA.118.039329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greiser M, Kerfant B-G, Williams GSB, Voigt N, Harks E, Dibb KM, Giese A, Meszaros J, Verheule S, Ravens U, et al. Tachycardia-induced silencing of subcellular Ca2+ signaling in atrial myocytes. J Clin Invest. 2014;124:4759–4772. doi: 10.1172/JCI70102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Hurtado N, Knollmann BC. Calcium in atrial fibrillation - pulling the trigger or not? J Clin Invest. 2014;124:4684–4686. doi: 10.1172/JCI77986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisner D, Neher E, Taschenberger H, Smith G. Physiology of intracellular calcium buffering. Physiol Rev. 2023;103:2767–2845. doi: 10.1152/physrev.00042.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke L, Qi XY, Dijkhuis A-J, Chartier D, Nattel S, Henning RH, Kampinga HH, Brundel BJJM. Calpain mediates cardiac troponin degradation and contractile dysfunction in atrial fibrillation. J Mol Cell Cardiol. 2008;45:685–693. doi: 10.1016/j.yjmcc.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 15.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4 [DOI] [PubMed] [Google Scholar]
- 16.Eiras S, Narolska NA, van Loon RB, Boontje NM, Zaremba R, Jimenez CR, Visser FC, Stooker W, van der Velden J, Stienen GJM. Alterations in contractile protein composition and function in human atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2006;41:467–477. doi: 10.1016/j.yjmcc.2006.06.072 [DOI] [PubMed] [Google Scholar]
- 17.Belus A, Piroddi N, Ferrantini C, Tesi C, Cazorla O, Toniolo L, Drost M, Mearini G, Carrier L, Rossi A, et al. Effects of chronic atrial fibrillation on active and passive force generation in human atrial myofibrils. Circ Res. 2010;107:144–152. doi: 10.1161/CIRCRESAHA.110.220699 [DOI] [PubMed] [Google Scholar]
- 18.Voigt N, Zhou XB, Dobrev D. Isolation of human atrial myocytes for simultaneous measurements of Ca2+ transients and membrane currents. J Vis Exp. 2013;77:e50235. doi: 10.3791/50235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, Henze S, Stauske M, Salinas G, Zimmermann W-H, et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. 2018;3:e99941. doi: 10.1172/jci.insight.99941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seibertz F, Rubio T, Springer R, Popp F, Ritter M, Liutkute A, Bartelt L, Stelzer L, Haghighi F, Pietras J, et al. Atrial fibrillation-associated electrical remodelling in human induced pluripotent stem cell-derived atrial cardiomyocytes: a novel pathway for antiarrhythmic therapy development. Cardiovasc Res. 2023;119:2623–2637. doi: 10.1093/cvr/cvad143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rössler U, Hennig AF, Stelzer N, Bose S, Kopp J, Søe K, Cyganek L, Zifarelli G, Ali S, von der Hagen M, et al. Efficient generation of osteoclasts from human induced pluripotent stem cells and functional investigations of lethal CLCN7-related osteopetrosis. J Bone Miner Res. 2021;36:1621–1635. doi: 10.1002/jbmr.4322 [DOI] [PubMed] [Google Scholar]
- 22.Hwang HS, Kryshtal DO, Feaster TK, Sánchez-Freire V, Zhang J, Kamp TJ, Hong CC, Wu JC, Knollmann BC. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. J Mol Cell Cardiol. 2015;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung P, Seibertz F, Fakuade FE, Ignatyeva N, Sampathkumar S, Ritter M, Li H, Mason FE, Ebert A, Voigt N. Increased cytosolic calcium buffering contributes to a cellular arrhythmogenic substrate in iPSC-cardiomyocytes from patients with dilated cardiomyopathy. Basic Res Cardiol. 2022;117:5. doi: 10.1007/s00395-022-00912-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seibertz F, Reynolds M, Voigt N. Single-cell optical action potential measurement in human induced pluripotent stem cell-derived cardiomyocytes. J Vis Exp. 2020;166:e61890. doi: 10.3791/61890 [DOI] [PubMed] [Google Scholar]
- 25.Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol. 2009;46:463–473. doi: 10.1016/j.yjmcc.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 26.Morano I, Hofmann F, Zimmer M, Rüegg JC. The influence of P-light chain phosphorylation by myosin light chain kinase on the calcium sensitivity of chemically skinned heart fibres. FEBS Lett. 1985;189:221–224. doi: 10.1016/0014-5793(85)81027-9 [DOI] [PubMed] [Google Scholar]
- 27.Fabiato A, Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972;31:293–307. doi: 10.1161/01.res.31.3.293 [DOI] [PubMed] [Google Scholar]
- 28.Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H2183–H2192. doi: 10.1152/ajpheart.00520.2005 [DOI] [PubMed] [Google Scholar]
- 29.Seibertz F, Sutanto H, Dülk R, Pronto JRD, Springer R, Rapedius M, Liutkute A, Ritter M, Jung P, Stelzer L, et al. Electrophysiological and calcium-handling development during long-term culture of human-induced pluripotent stem cell-derived cardiomyocytes. Basic Res Cardiol. 2023;118:14. doi: 10.1007/s00395-022-00973-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruegmann T, Beiert T, Vogt CC, Schrickel JW, Sasse P. Optogenetic termination of atrial fibrillation in mice. Cardiovasc Res. 2018;114:713–723. doi: 10.1093/cvr/cvx250 [DOI] [PubMed] [Google Scholar]
- 31.Trafford AW, Díaz ME, Eisner DA. A novel, rapid and reversible method to measure Ca2+ buffering and time-course of total sarcoplasmic reticulum Ca2+ content in cardiac ventricular myocytes. Pflugers Arch. 1999;437:501–503. doi: 10.1007/s004240050808 [DOI] [PubMed] [Google Scholar]
- 32.Díaz ME, Trafford AW, Eisner DA. The effects of exogenous calcium buffers on the systolic calcium transient in rat ventricular myocytes. Biophys J. 2001;80:1915–1925. doi: 10.1016/S0006-3495(01)76161-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28 [DOI] [PubMed] [Google Scholar]
- 34.Heijman J, Muna AP, Veleva T, Molina CE, Sutanto H, Tekook M, Wang Q, Abu-Taha IH, Gorka M, Künzel S, et al. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ Res. 2020;127:1036–1055. doi: 10.1161/CIRCRESAHA.120.316710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res. 2011;109:1055–1066. doi: 10.1161/CIRCRESAHA.111.253955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Nattel S, Yue L, Gaspo R, Feng J, Li G-R. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 2012;81:512–525. [DOI] [PubMed] [Google Scholar]
- 37.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428 [DOI] [PubMed] [Google Scholar]
- 38.Christ T, Boknik P, Wöhrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A [DOI] [PubMed] [Google Scholar]
- 39.Aimé-Sempé C, Folliguet T, Rücker-Martin C, Krajewska M, Krajewska S, Heimburger M, Aubier M, Mercadier JJ, Reed JC, Hatem SN. Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol. 1999;34:1577–1586. doi: 10.1016/s0735-1097(99)00382-4 [DOI] [PubMed] [Google Scholar]
- 40.Schotten U, Ausma J, Stellbrink C, Sabatschus I, Vogel M, Frechen D, Schoendube F, Hanrath P, Allessie MA. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation. 2001;103:691–698. doi: 10.1161/01.cir.103.5.691 [DOI] [PubMed] [Google Scholar]
- 41.Logan WF, Rowlands DJ, Howitt G, Holmes AM. Left atrial activity following cardioversion. Lancet. 1965;2:471–473. doi: 10.1016/s0140-6736(65)91427-3 [DOI] [PubMed] [Google Scholar]
- 42.Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D’hooge J, Heidbüchel H, Sipido KR, Willems R. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res. 2009;105:876–885. doi: 10.1161/CIRCRESAHA.109.206276 [DOI] [PubMed] [Google Scholar]
- 43.Li N, Brundel BJJM. Inflammasomes and proteostasis novel molecular mechanisms associated with atrial fibrillation. Circ Res. 2020;127:73–90. doi: 10.1161/CIRCRESAHA.119.316364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rennison JH, Li L, Lin CR, Lovano BS, Castel L, Wass SY, Cantlay CC, McHale M, Gillinov AM, Mehra R, et al. Atrial fibrillation rhythm is associated with marked changes in metabolic and myofibrillar protein expression in left atrial appendage. Pflugers Arch. 2021;473:461–475. doi: 10.1007/s00424-021-02514-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makary S, Voigt N, Maguy A, Wakili R, Nishida K, Harada M, Dobrev D, Nattel S. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circ Res. 2011;109:1031–1043. doi: 10.1161/CIRCRESAHA.111.253120 [DOI] [PubMed] [Google Scholar]
- 46.Morano I. Tuning the human heart molecular motors by myosin light chains. J Mol Med (Berl). 1999;77:544–555. doi: 10.1007/s001099900031 [DOI] [PubMed] [Google Scholar]
- 47.Kockskämper J, Khafaga M, Grimm M, Elgner A, Walther S, Kockskämper A, von Lewinski D, Post H, Grossmann M, Dörge H, et al. Angiotensin II and myosin light-chain phosphorylation contribute to the stretch-induced slow force response in human atrial myocardium. Cardiovasc Res. 2008;79:642–651. doi: 10.1093/cvr/cvn126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845 [DOI] [PubMed] [Google Scholar]
- 49.Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol. 2014;76:107–127. doi: 10.1146/annurev-physiol-020911-153308 [DOI] [PubMed] [Google Scholar]
- 50.Macquaide N, Tuan H-TM, Hotta J-I, Sempels W, Lenaerts I, Holemans P, Hofkens J, Jafri MS, Willems R, Sipido KR. Ryanodine receptor cluster fragmentation and redistribution in persistent atrial fibrillation enhance calcium release. Cardiovasc Res. 2015;108:387–398. doi: 10.1093/cvr/cvv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutanto H, van Sloun B, Schönleitner P, van Zandvoort MAMJ, Antoons G, Heijman J. The subcellular distribution of ryanodine receptors and L-type Ca2+ channels modulates Ca2+-transient properties and spontaneous Ca2+-release events in atrial cardiomyocytes. Front Physiol. 2018;9:1108. doi: 10.3389/fphys.2018.01108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keizer J, Smith GD, Ponce-Dawson S, Pearson JE. Saltatory propagation of Ca2+ waves by Ca2+ sparks. Biophys J. 1998;75:595–600. doi: 10.1016/S0006-3495(98)77550-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bovo E, Mazurek SR, Fill M, Zima AV. Cytosolic Ca2+ buffering determines the intra-SR Ca2+ concentration at which cardiac Ca2+ sparks terminate. Cell Calcium. 2015;58:246–253. doi: 10.1016/j.ceca.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ausma J, Litjens N, Lenders MH, Duimel H, Mast F, Wouters L, Ramaekers F, Allessie M, Borgers M. Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J Mol Cell Cardiol. 2001;33:2083–2094. doi: 10.1006/jmcc.2001.1472 [DOI] [PubMed] [Google Scholar]
- 56.Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, Lambing J, Lee J, Semigran M, Sehnert AJ. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170:741–748. doi: 10.7326/M18-3016 [DOI] [PubMed] [Google Scholar]
- 57.Masri A, Olivotto I. Cardiac myosin inhibitors as a novel treatment option for obstructive hypertrophic cardiomyopathy: addressing the core of the matter. J Am Heart Assoc. 2022;11:e024656. doi: 10.1161/JAHA.121.024656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zile MA, Trayanova NA. Increased thin filament activation enhances alternans in human chronic atrial fibrillation. Am J Physiol Heart Circ Physiol. 2018;315:H1453–H1462. doi: 10.1152/ajpheart.00658.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA. 2003;100:13716–13721. doi: 10.1073/pnas.2235652100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mason FE, Pronto JRD, Alhussini K, Maack C, Voigt N. Cellular and mitochondrial mechanisms of atrial fibrillation. Basic Res Cardiol. 2020;115:72. doi: 10.1007/s00395-020-00827-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams GSB, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochondrial calcium uptake. Proc Natl Acad Sci USA. 2013;110:10479–10486. doi: 10.1073/pnas.1300410110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamilton S, Terentyeva R, Clements RT, Belevych AE, Terentyev D. Sarcoplasmic reticulum-mitochondria communication; implications for cardiac arrhythmia. J Mol Cell Cardiol. 2021;156:105–113. doi: 10.1016/j.yjmcc.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci. 2004;117:6327–6337. doi: 10.1242/jcs.01559 [DOI] [PubMed] [Google Scholar]
- 64.Lu X, Ginsburg KS, Kettlewell S, Bossuyt J, Smith GL, Bers DM. Measuring local gradients of intramitochondrial [Ca2+] in cardiac myocytes during sarcoplasmic reticulum Ca2+ release. Circ Res. 2013;112:424–431. doi: 10.1161/CIRCRESAHA.111.300501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, O’Rourke B, Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047 [DOI] [PubMed] [Google Scholar]
- 68.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. doi: 10.1161/CIRCULATIONAHA.113.006611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi XY, Yeh Y-H, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJJM, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res. 2008;103:845–854. doi: 10.1161/CIRCRESAHA.108.175463 [DOI] [PubMed] [Google Scholar]
- 70.Abacilar AF, Dogan OF. Levosimendan use decreases atrial fibrillation in patients after coronary artery bypass grafting: a pilot study. Heart Surg Forum. 2013;16:E287–E294. doi: 10.1532/hsf98.2013190 [DOI] [PubMed] [Google Scholar]
- 71.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eley DW, Lake N, Ter Keurs HEDJ. Taurine depletion and excitation-contraction coupling in rat myocardium. Circ Res. 1994;74:1210–1219. [DOI] [PubMed] [Google Scholar]
- 73.Creighton JV, de Souza Gonçalves L, Artioli GG, Tan D, Elliott-Sale KJ, Turner MD, Doig CL, Sale C. Physiological roles of carnosine in myocardial function and health. Adv Nutr. 2022;13:1914–1929. doi: 10.1093/advances/nmac059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seibertz F, Rapedius M, Fakuade FE, Tomsits P, Liutkute A, Cyganek L, Becker N, Majumder R, Clauß S, Fertig N, et al. A modern automated patch-clamp approach for high throughput electrophysiology recordings in native cardiomyocytes. Commun Biol. 2022;5:969. doi: 10.1038/s42003-022-03871-2 [DOI] [PMC free article] [PubMed] [Google Scholar]