Abstract
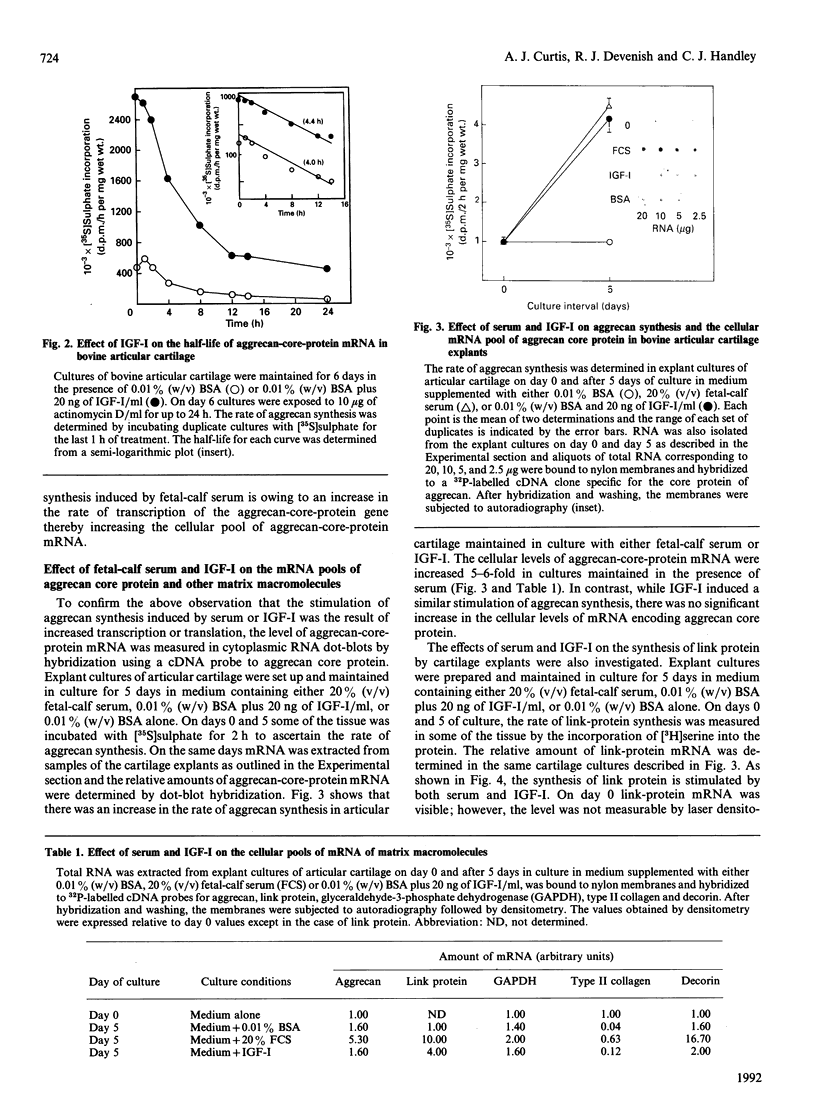
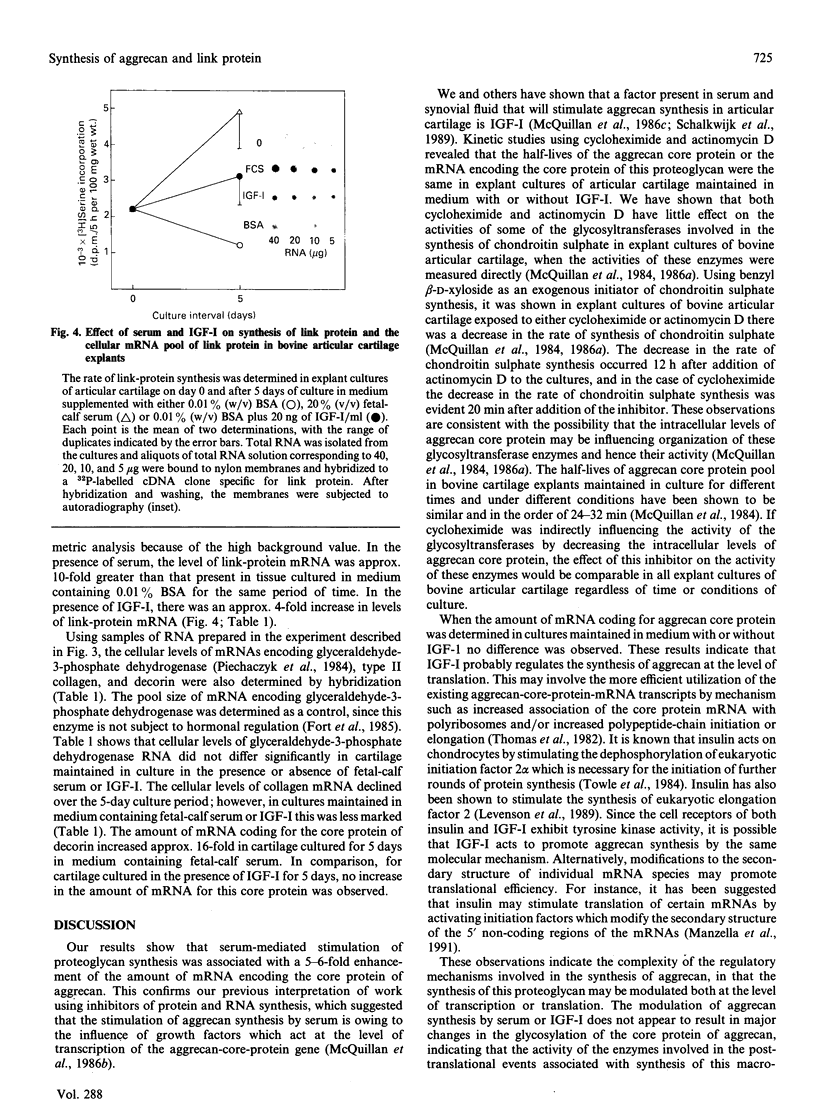
The addition of serum or insulin-like growth factor-I (IGF-I) to the medium of explant cultures of bovine articular cartilage is known to stimulate the synthesis of aggrecan in a dose-dependent manner. The half-life of the pool of proteoglycan core protein was measured in adult articular cartilage cultured for 6 days in the presence and absence of 20 ng of IGF-I/ml and shown to be 24 min under both sets of conditions. The half-life of the mRNA pool coding for aggrecan was also determined and shown to be approx. 4 h in cartilage maintained in culture with or without IGF-I. The pool size of mRNA coding for aggrecan core protein increased 5-6-fold in cartilage explants maintained in culture in medium containing 20% (v/v) fetal-calf serum; however, in tissue maintained with medium containing IGF-I there was no increase in the cellular levels of this mRNA. This suggests that aggrecan synthesis is stimulated by IGF-I at the level of translation of mRNA coding for the core protein of this proteoglycan and that other growth factors are present in serum that stimulate aggrecan synthesis at the level of transcription of the core-protein gene. Inclusion of serum or IGF-I in the medium of cartilage explant cultures induced increases in the amounts of mRNA coding for type II collagen and link protein, whereas only serum enhanced the amount of mRNA for the core protein of decorin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis A. J., Ng C. K., Handley C. J., Robinson H. C. Effect of insulin-like growth factor-I on the synthesis and distribution of link protein and hyaluronan in explant cultures of articular cartilage. Biochim Biophys Acta. 1992 Jun 29;1135(3):309–317. doi: 10.1016/0167-4889(92)90236-5. [DOI] [PubMed] [Google Scholar]
- Doege K., Hassell J. R., Caterson B., Yamada Y. Link protein cDNA sequence reveals a tandemly repeated protein structure. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3761–3765. doi: 10.1073/pnas.83.11.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Extracellular matrix metabolism by chondrocytes. III. Modulation of proteoglycan synthesis by extracellular levels of proteoglycan in cartilage cells in culture. Biochim Biophys Acta. 1977 Nov 7;500(1):132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Handley C. J., McQuillan D. J., Hascall G. K., Robinson H. C., Lowther D. A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
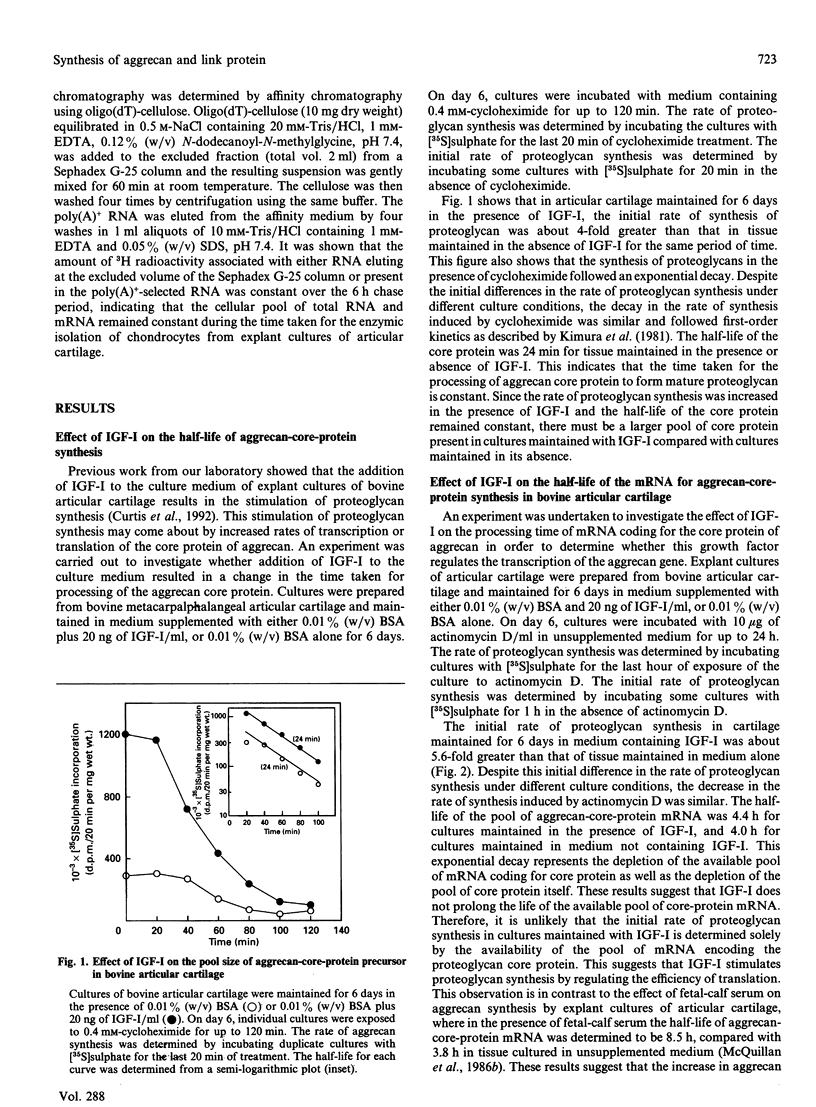
- Kimura J. H., Caputo C. B., Hascall V. C. The effect of cycloheximide on synthesis of proteoglycans by cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 May 10;256(9):4368–4376. [PubMed] [Google Scholar]
- Kimura J. H., Hardingham T. E., Hascall V. C. Assembly of newly synthesized proteoglycan and link protein into aggregates in cultures of chondrosarcoma chondrocytes. J Biol Chem. 1980 Aug 10;255(15):7134–7143. [PubMed] [Google Scholar]
- Leboy P. S., Shapiro I. M., Uschmann B. D., Oshima O., Lin D. Gene expression in mineralizing chick epiphyseal cartilage. J Biol Chem. 1988 Jun 15;263(17):8515–8520. [PubMed] [Google Scholar]
- Levenson R. M., Nairn A. C., Blackshear P. J. Insulin rapidly induces the biosynthesis of elongation factor 2. J Biol Chem. 1989 Jul 15;264(20):11904–11911. [PubMed] [Google Scholar]
- Luyten F. P., Hascall V. C., Nissley S. P., Morales T. I., Reddi A. H. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988 Dec;267(2):416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- Manzella J. M., Rychlik W., Rhoads R. E., Hershey J. W., Blackshear P. J. Insulin induction of ornithine decarboxylase. Importance of mRNA secondary structure and phosphorylation of eucaryotic initiation factors eIF-4B and eIF-4E. J Biol Chem. 1991 Feb 5;266(4):2383–2389. [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Campbell M. A., Bolis S., Milway V. E., Herington A. C. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986 Dec 1;240(2):423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C. Control of proteoglycan biosynthesis. Further studies on the effect of serum on cultured bovine articular cartilage. Biochem J. 1986 Aug 1;237(3):741–747. doi: 10.1042/bj2370741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C., Ng K., Tzaicos C., Brooks P. R., Lowther D. A. The relation of protein synthesis to chondroitin sulphate biosynthesis in cultured bovine cartilage. Biochem J. 1984 Dec 15;224(3):977–988. doi: 10.1042/bj2240977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C., Ng K., Tzaicos C. The relation of RNA synthesis to chondroitin sulphate biosynthesis in cultured bovine cartilage. Biochem J. 1986 Apr 15;235(2):499–505. doi: 10.1042/bj2350499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Kraft E. L., Jourdian G. W., Van Valen T. R. Phosphorylation of chondroitin sulfate in proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1984 Feb 10;259(3):1720–1726. [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk J., Joosten L. A., van den Berg W. B., van Wyk J. J., van de Putte L. B. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum. 1989 Jan;32(1):66–71. doi: 10.1002/anr.1780320111. [DOI] [PubMed] [Google Scholar]
- Su M. W., Lee B., Ramirez F., Machado M., Horton W. Nucleotide sequence of the full length cDNA encoding for human type II procollagen. Nucleic Acids Res. 1989 Nov 25;17(22):9473–9473. doi: 10.1093/nar/17.22.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch G. H., Handley C. J., Cornell H. J., Herington A. C. Effects of free and bound insulin-like growth factors on proteoglycan metabolism in articular cartilage explants. J Orthop Res. 1992 Jan;10(1):14–22. doi: 10.1002/jor.1100100103. [DOI] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Towle C. A., Mankin H. J., Avruch J., Treadwell B. V. Insulin promoted decrease in the phosphorylation of protein synthesis initiation factor eIF-2. Biochem Biophys Res Commun. 1984 May 31;121(1):134–140. doi: 10.1016/0006-291x(84)90697-1. [DOI] [PubMed] [Google Scholar]