Abstract
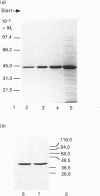
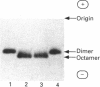
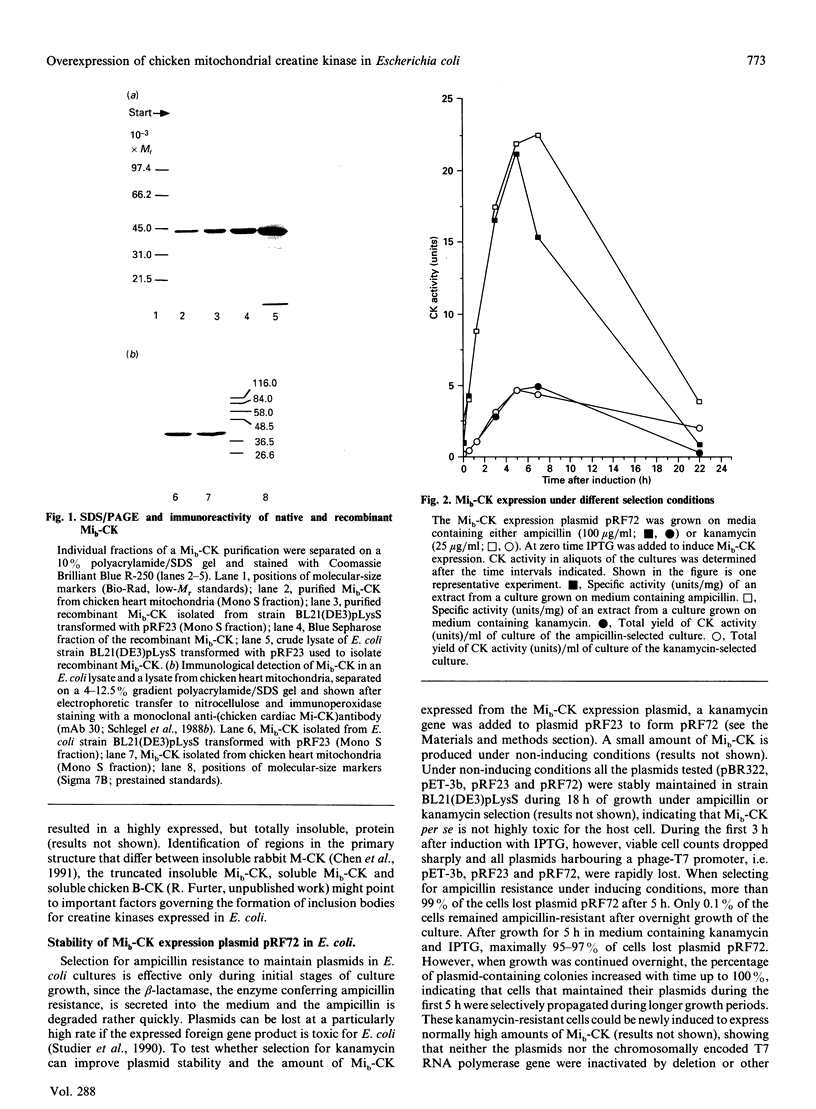
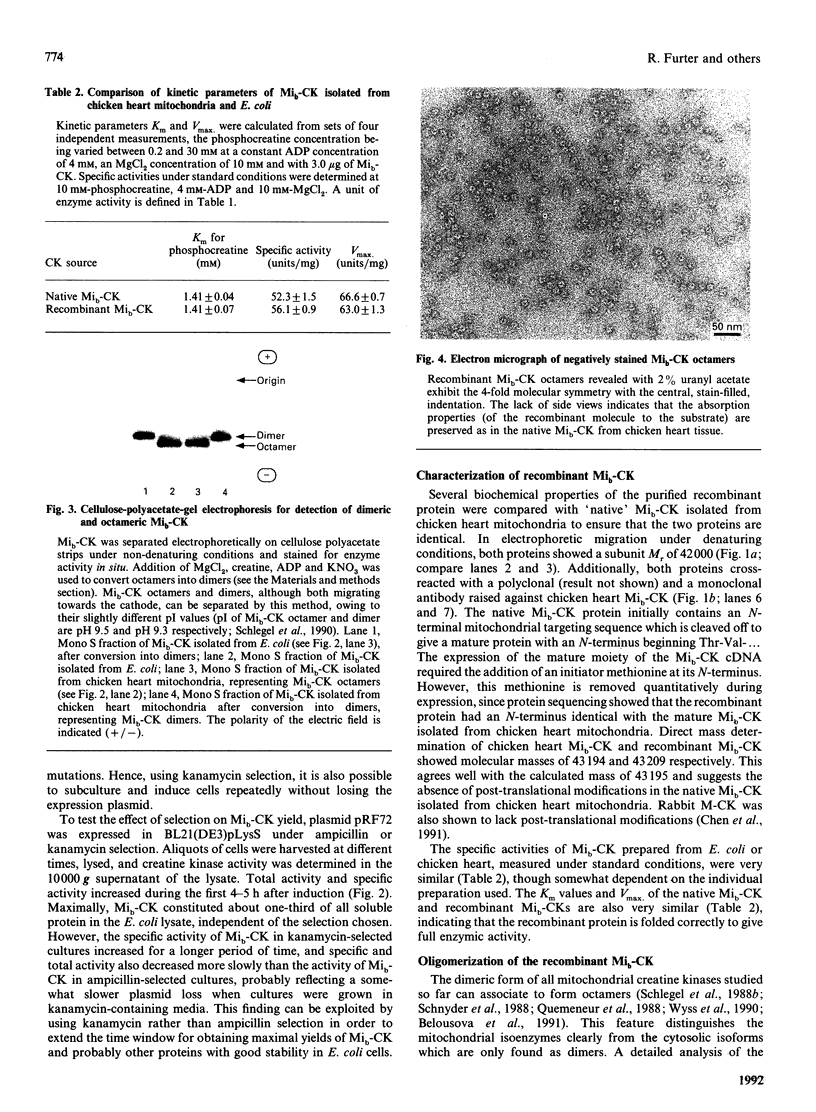
Sarcomeric mitochondrial creatine kinase (Mib-CK) of chicken was expressed in Escherichia coli as a soluble enzyme by using an inducible phage-T7 promoter. Up to one third of the protein in E. coli extracts consisted of soluble recombinant Mib-CK in an enzymically active form. Approx. 20 mg of nearly-homogenous Mib-CK was isolated in a two-step isolation procedure starting with 1 litre of isopropyl beta-D-thiogalactopyranoside-induced E. coli culture, whereas previous attempts to express other CK genes in E. coli have resulted in 20-fold lower yields and inclusion-body formation. Selection of the Mib-CK expression plasmid on media containing kanamycin rather than ampicillin extended the time period of maximal Mib-CK expression. Recombinant Mib-CK displayed an identical N-terminal amino acid sequence, identical Km for phosphocreatine and Vmax. values, the same electrophoretic behaviour and the same immunological cross-reactivity as the native enzyme isolated from chicken heart mitochondria. The recombinant Mib-CK had the same molecular mass as native chicken Mib-CK in m.s. analysis, indicating that post-translational modification of the enzyme in chicken tissue does not occur. As judged by gel-permeation chromatography and electron microscopy, recombinant enzyme formed predominantly octameric oligomers with the same overall structure as the chicken heart enzyme. Furthermore, the enzymes isolated from both sources formed protein crystals of space group P42(1)2, when grown in the absence of ATP, with one Mi-CK octamer per asymmetric unit. The indistinguishable X-ray-diffraction patterns indicate identical structures for the native and recombinant proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams V., Bosch W., Schlegel J., Wallimann T., Brdiczka D. Further characterization of contact sites from mitochondria of different tissues: topology of peripheral kinases. Biochim Biophys Acta. 1989 Jun 6;981(2):213–225. doi: 10.1016/0005-2736(89)90031-x. [DOI] [PubMed] [Google Scholar]
- Babbitt P. C., West B. L., Buechter D. D., Kuntz I. D., Kenyon G. L. Removal of a proteolytic activity associated with aggregates formed from expression of creatine kinase in Escherichia coli leads to improved recovery of active enzyme. Biotechnology (N Y) 1990 Oct;8(10):945–949. doi: 10.1038/nbt1090-945. [DOI] [PubMed] [Google Scholar]
- Belousova L. V., Fedosov S. N., Orlova E. V., Stel'mashchuk VYa The structural features of beef heart mitochondrial creatine kinase. Biochem Int. 1991 May;24(1):51–58. [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981 Jan 30;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brdiczka D. Contact sites between mitochondrial envelope membranes. Structure and function in energy- and protein-transfer. Biochim Biophys Acta. 1991 Nov 13;1071(3):291–312. doi: 10.1016/0304-4157(91)90018-r. [DOI] [PubMed] [Google Scholar]
- Chen L. H., Babbitt P. C., Vásquez J. R., West B. L., Kenyon G. L. Cloning and expression of functional rabbit muscle creatine kinase in Escherichia coli. Addressing the problem of microheterogeneity. J Biol Chem. 1991 Jun 25;266(18):12053–12057. [PubMed] [Google Scholar]
- Eppenberger H. M., Perriard J. C., Wallimann T. Analysis of creatine kinase isozymes during muscle differentiation. Isozymes Curr Top Biol Med Res. 1983;7:19–38. [PubMed] [Google Scholar]
- Haas R. C., Strauss A. W. Separate nuclear genes encode sarcomere-specific and ubiquitous human mitochondrial creatine kinase isoenzymes. J Biol Chem. 1990 Apr 25;265(12):6921–6927. [PubMed] [Google Scholar]
- Hossle J. P., Schlegel J., Wegmann G., Wyss M., Böhlen P., Eppenberger H. M., Wallimann T., Perriard J. C. Distinct tissue specific mitochondrial creatine kinases from chicken brain and striated muscle with a conserved CK framework. Biochem Biophys Res Commun. 1988 Feb 29;151(1):408–416. doi: 10.1016/0006-291x(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Heldt H. W., Klingenberg M. High activity of creatine kinase in mitochondria from muscle and brain and evidence for a separate mitochondrial isoenzyme of creatine kinase. Biochem Biophys Res Commun. 1964 Aug 11;16(6):516–521. doi: 10.1016/0006-291x(64)90185-8. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Lehninger A. L. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem. 1973 Jul 10;248(13):4803–4810. [PubMed] [Google Scholar]
- James P., Wyss M., Lutsenko S., Wallimann T., Carafoli E. ATP binding site of mitochondrial creatine kinase. Affinity labelling of Asp-335 with C1RATP. FEBS Lett. 1990 Oct 29;273(1-2):139–143. doi: 10.1016/0014-5793(90)81069-z. [DOI] [PubMed] [Google Scholar]
- Klein S. C., Haas R. C., Perryman M. B., Billadello J. J., Strauss A. W. Regulatory element analysis and structural characterization of the human sarcomeric mitochondrial creatine kinase gene. J Biol Chem. 1991 Sep 25;266(27):18058–18065. [PubMed] [Google Scholar]
- Mariman E. C., Broers C. A., Claesen C. A., Tesser G. I., Wieringa B. Structure and expression of the human creatine kinase B gene. Genomics. 1987 Oct;1(2):126–137. doi: 10.1016/0888-7543(87)90004-8. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Payne R. M., Haas R. C., Strauss A. W. Structural characterization and tissue-specific expression of the mRNAs encoding isoenzymes from two rat mitochondrial creatine kinase genes. Biochim Biophys Acta. 1991 Jul 23;1089(3):352–361. doi: 10.1016/0167-4781(91)90176-m. [DOI] [PubMed] [Google Scholar]
- Quemeneur E., Eichenberger D., Goldschmidt D., Vial C., Beauregard G., Potier M. The radiation inactivation method provides evidence that membrane-bound mitochondrial creatine kinase is an oligomer. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1310–1314. doi: 10.1016/s0006-291x(88)81371-8. [DOI] [PubMed] [Google Scholar]
- Rojo M., Hovius R., Demel R. A., Nicolay K., Wallimann T. Mitochondrial creatine kinase mediates contact formation between mitochondrial membranes. J Biol Chem. 1991 Oct 25;266(30):20290–20295. [PubMed] [Google Scholar]
- Saks V. A., Kuznetsov A. V., Kupriyanov V. V., Miceli M. V., Jacobus W. E. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane-matrix preparation. J Biol Chem. 1985 Jun 25;260(12):7757–7764. [PubMed] [Google Scholar]
- Schlegel J., Wyss M., Eppenberger H. M., Wallimann T. Functional studies with the octameric and dimeric form of mitochondrial creatine kinase. Differential pH-dependent association of the two oligomeric forms with the inner mitochondrial membrane. J Biol Chem. 1990 Jun 5;265(16):9221–9227. [PubMed] [Google Scholar]
- Schlegel J., Wyss M., Schürch U., Schnyder T., Quest A., Wegmann G., Eppenberger H. M., Wallimann T. Mitochondrial creatine kinase from cardiac muscle and brain are two distinct isoenzymes but both form octameric molecules. J Biol Chem. 1988 Nov 15;263(32):16963–16969. [PubMed] [Google Scholar]
- Schlegel J., Zurbriggen B., Wegmann G., Wyss M., Eppenberger H. M., Wallimann T. Native mitochondrial creatine kinase forms octameric structures. I. Isolation of two interconvertible mitochondrial creatine kinase forms, dimeric and octameric mitochondrial creatine kinase: characterization, localization, and structure-function relationships. J Biol Chem. 1988 Nov 15;263(32):16942–16953. [PubMed] [Google Scholar]
- Schnyder T., Engel A., Lustig A., Wallimann T. Native mitochondrial creatine kinase forms octameric structures. II. Characterization of dimers and octamers by ultracentrifugation, direct mass measurements by scanning transmission electron microscopy, and image analysis of single mitochondrial creatine kinase octamers. J Biol Chem. 1988 Nov 15;263(32):16954–16962. [PubMed] [Google Scholar]
- Schnyder T., Sargent D. F., Richmond T. J., Eppenberger H. M., Wallimann T. Crystallization and preliminary X-ray analysis of two different forms of mitochondrial creatine kinase from chicken cardiac muscle. J Mol Biol. 1990 Dec 20;216(4):809–812. doi: 10.1016/S0022-2836(99)80002-3. [DOI] [PubMed] [Google Scholar]
- Schnyder T., Winkler H., Gross H., Eppenberger H. M., Wallimann T. Crystallization of mitochondrial creatine kinase. Growing of large protein crystals and electron microscopic investigation of microcrystals consisting of octamers. J Biol Chem. 1991 Mar 15;266(8):5318–5322. [PubMed] [Google Scholar]
- Scholte H. R., Weijers P. J., Wit-Peeters E. M. The localization of mitochondrial creatine kinase, and its use for the determination of the sidedness of submitochondrial particles. Biochim Biophys Acta. 1973 Feb 16;291(3):764–773. doi: 10.1016/0005-2736(73)90479-3. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Schlösser T., Eppenberger H. M. Function of M-line-bound creatine kinase as intramyofibrillar ATP regenerator at the receiving end of the phosphorylcreatine shuttle in muscle. J Biol Chem. 1984 Apr 25;259(8):5238–5246. [PubMed] [Google Scholar]
- Wallimann T., Schnyder T., Schlegel J., Wyss M., Wegmann G., Rossi A. M., Hemmer W., Eppenberger H. M., Quest A. F. Subcellular compartmentation of creatine kinase isoenzymes, regulation of CK and octameric structure of mitochondrial CK: important aspects of the phosphoryl-creatine circuit. Prog Clin Biol Res. 1989;315:159–176. [PubMed] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H. M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992 Jan 1;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M., Schlegel J., James P., Eppenberger H. M., Wallimann T. Mitochondrial creatine kinase from chicken brain. Purification, biophysical characterization, and generation of heterodimeric and heterooctameric molecules with subunits of other creatine kinase isoenzymes. J Biol Chem. 1990 Sep 15;265(26):15900–15908. [PubMed] [Google Scholar]