Abstract
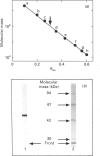
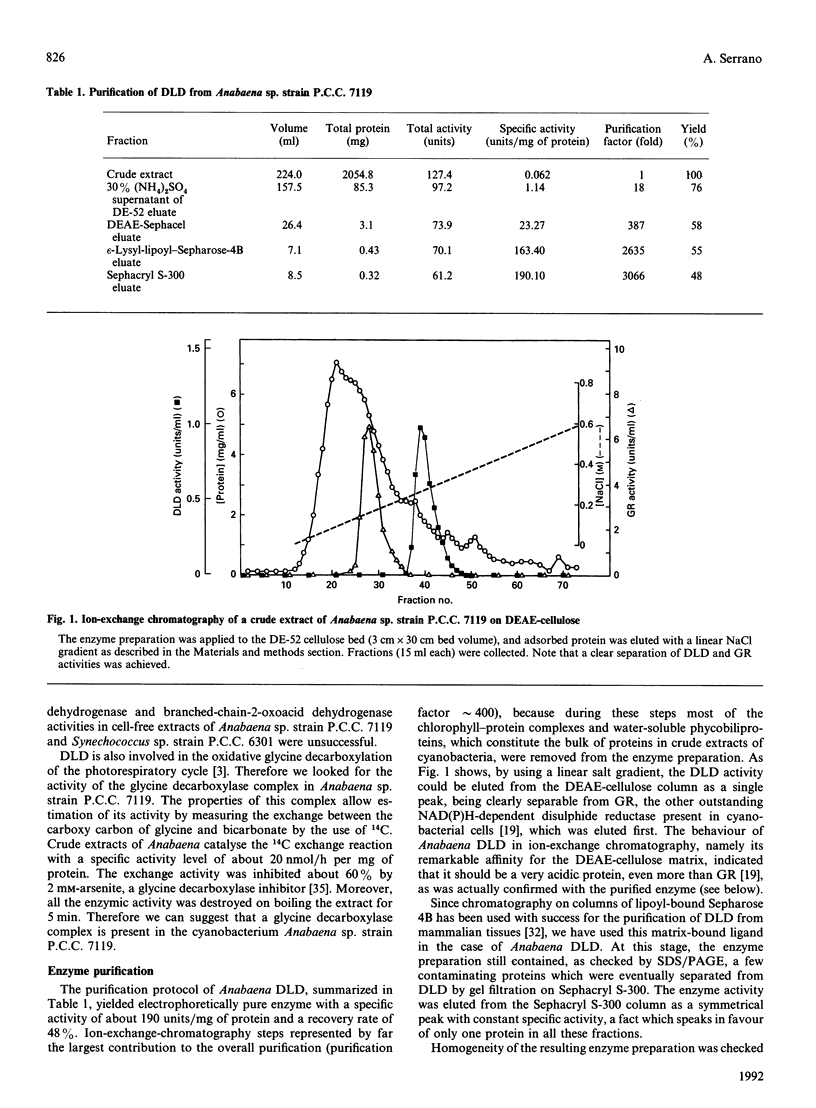
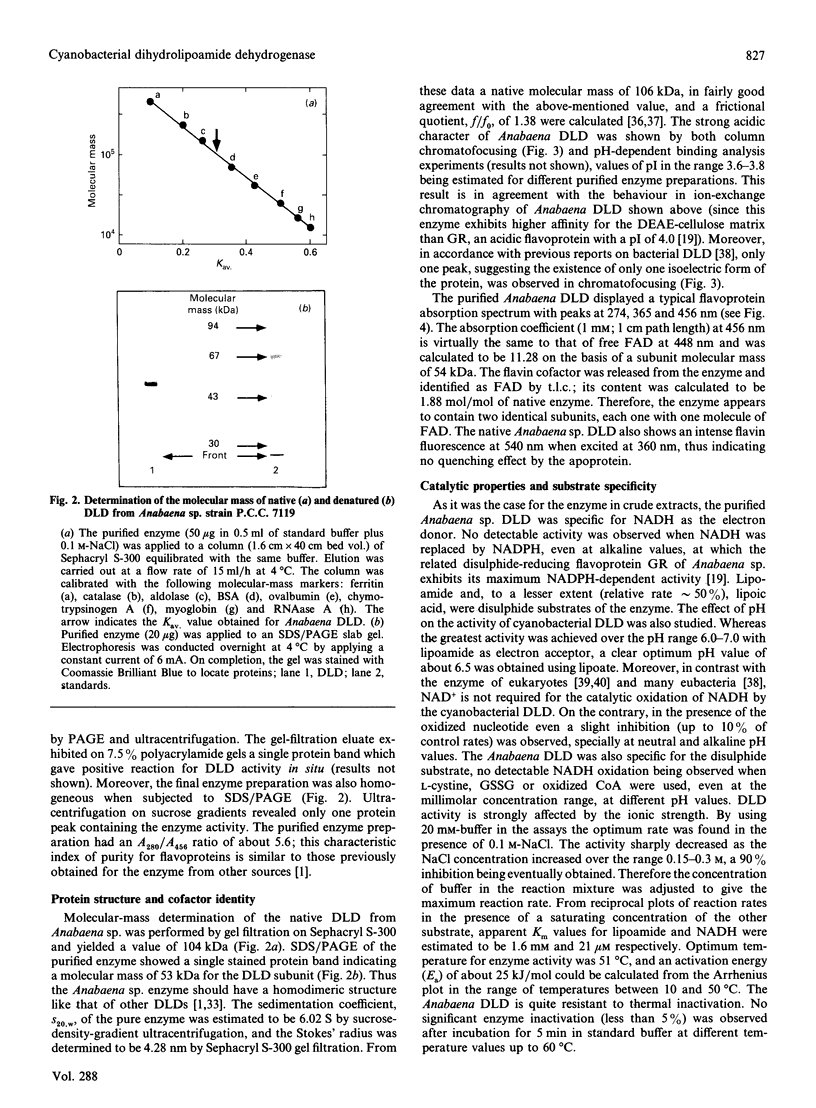
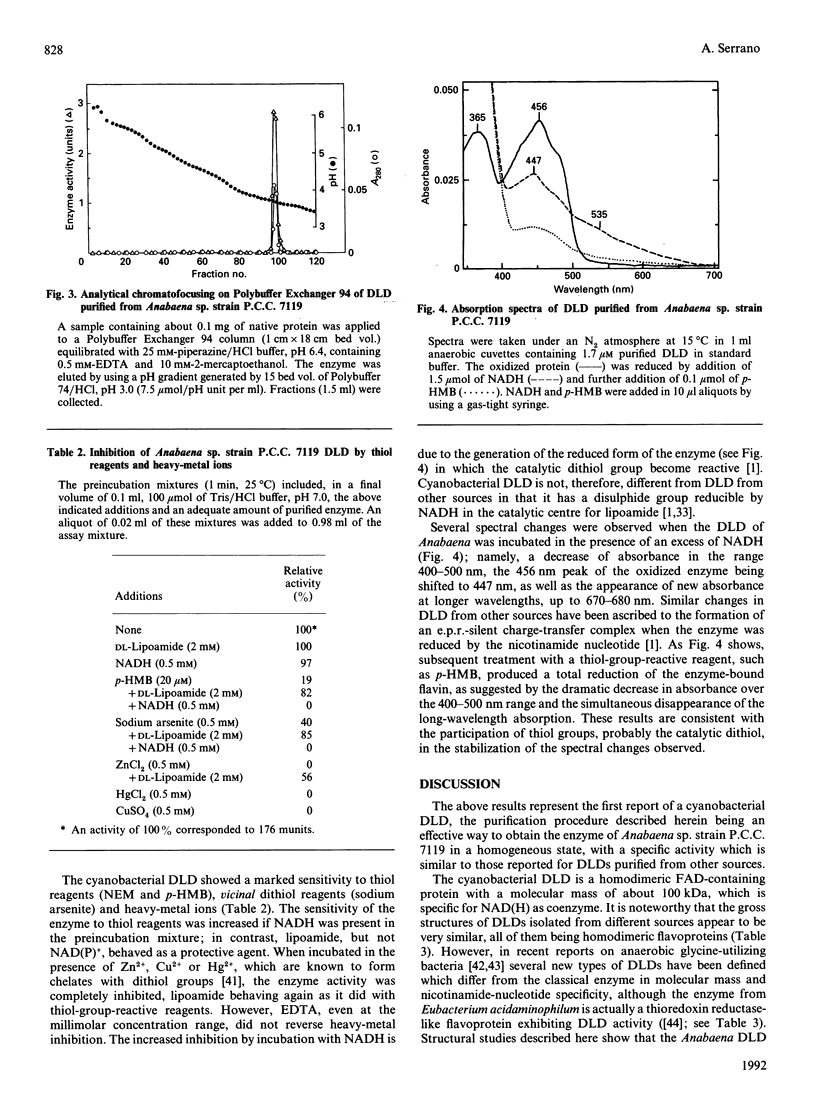
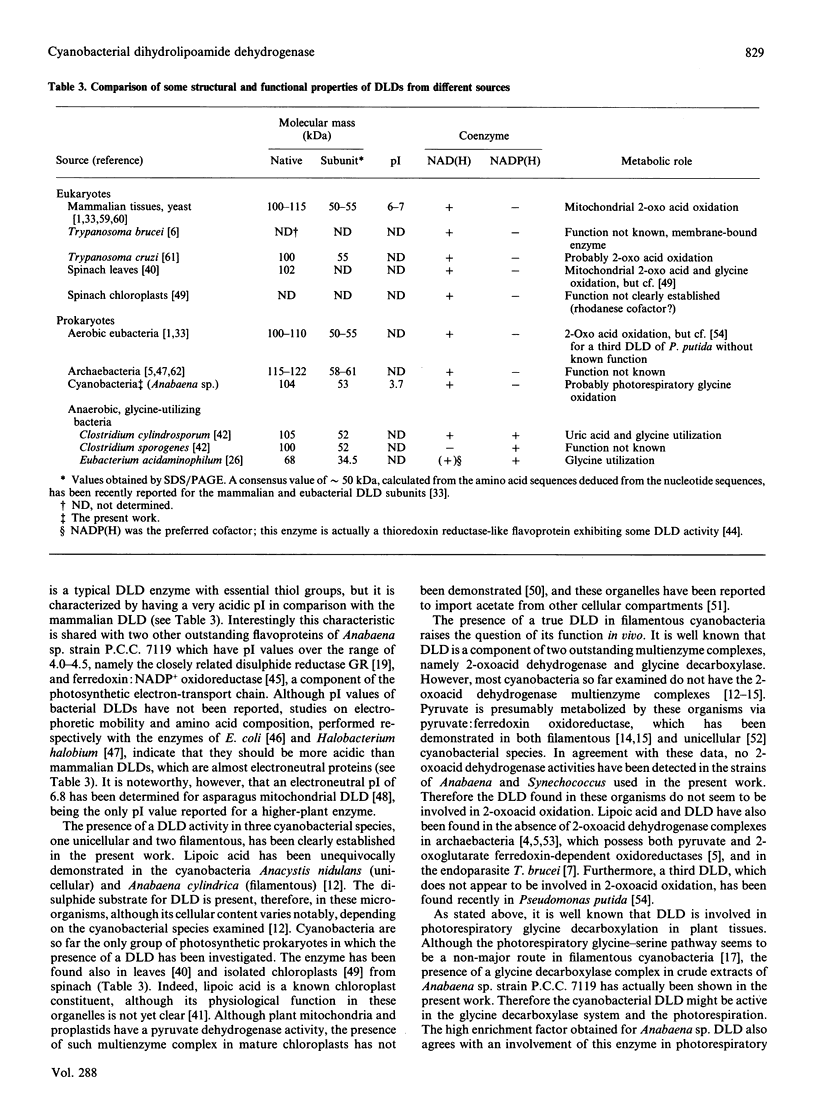
A dihydrolipoamide dehydrogenase (dihydrolipoamide: NAD+ oxidoreductase, EC 1.8.1.4) (DLD) has been found in the soluble fraction of cells of both unicellular (Synechococcus sp. strain P.C.C. 6301) and filamentous (Calothrix sp. strain P.C.C. 7601 and Anabaena sp. strain P.C.C. 7119) cyanobacteria. DLD from Anabaena sp. was purified 3000-fold to electrophoretic homogeneity. The purified enzyme exhibited a specific activity of 190 units/mg and was characterized as a dimeric FAD-containing protein with a native molecular mass of 104 kDa, a Stokes' radius of 4.28 nm and a very acidic pI value of about 3.7. As is the case with the same enzyme from other sources, cyanobacterial DLD showed specificity for NADH and lipoamide, or lipoic acid, as substrates. Nevertheless, the strong acidic character of the Anabaena DLD is a distinctive feature with respect to the same enzyme from other organisms. The presence of essential thiol groups was suggested by the inactivation produced by thiol-group-reactive reagents and heavy-metal ions, with lipoamide, but not NAD+, behaving as a protective agent. The function and physiological significance of Anabaena DLD are discussed in relation to the fact that 2-oxoacid dehydrogenase complexes have not been detected so far in filamentous cyanobacteria. Glycine decarboxylase activity, which might be involved in photorespiratory metabolism, has been found, however, in cell extracts of Anabaena sp. strain P.C.C. 7119 as the present study demonstrates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bothe H., Falkenberg B., Nolteernsting U. Properties and function of the pyruvate: ferredoxin oxidoreductase from the blue-green alga Anabaena cylindrica. Arch Mikrobiol. 1974 Mar 28;96(4):291–304. doi: 10.1007/BF00590185. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burns G., Sykes P. J., Hatter K., Sokatch J. R. Isolation of a third lipoamide dehydrogenase from Pseudomonas putida. J Bacteriol. 1989 Feb;171(2):665–668. doi: 10.1128/jb.171.2.665-668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers D. J., Pons G., Patel M. S. Dihydrolipoamide dehydrogenase: functional similarities and divergent evolution of the pyridine nucleotide-disulfide oxidoreductases. Arch Biochem Biophys. 1989 Feb 1;268(2):409–425. doi: 10.1016/0003-9861(89)90309-3. [DOI] [PubMed] [Google Scholar]
- Codd G. A., Stewart W. D. Pathways of glycollate metabolism in the blue-green alga Anabaena cylindrica. Arch Mikrobiol. 1973 Dec 4;94(1):11–28. doi: 10.1007/BF00414075. [DOI] [PubMed] [Google Scholar]
- Cohn M. L., Wang L., Scouten W., McManus I. R. Intramitochondrial distribution of multiple forms of pig heart lipoamide dehydrogenase. Biochim Biophys Acta. 1968 Apr 24;159(1):182–185. doi: 10.1016/0005-2744(68)90258-1. [DOI] [PubMed] [Google Scholar]
- Danson M. J. Archaebacteria: the comparative enzymology of their central metabolic pathways. Adv Microb Physiol. 1988;29:165–231. doi: 10.1016/s0065-2911(08)60348-3. [DOI] [PubMed] [Google Scholar]
- Danson M. J., Conroy K., McQuattie A., Stevenson K. J. Dihydrolipoamide dehydrogenase from Trypanosoma brucei. Characterization and cellular location. Biochem J. 1987 May 1;243(3):661–665. doi: 10.1042/bj2430661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson M. J. Dihydrolipoamide dehydrogenase: a 'new' function for an old enzyme? Biochem Soc Trans. 1988 Apr;16(2):87–89. doi: 10.1042/bst0160087. [DOI] [PubMed] [Google Scholar]
- Dietrichs D., Andreesen J. R. Purification and comparative studies of dihydrolipoamide dehydrogenases from the anaerobic, glycine-utilizing bacteria Peptostreptococcus glycinophilus, Clostridium cylindrosporum, and Clostridium sporogenes. J Bacteriol. 1990 Jan;172(1):243–251. doi: 10.1128/jb.172.1.243-251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichs D., Meyer M., Schmidt B., Andreesen J. R. Purification of NADPH-dependent electron-transferring flavoproteins and N-terminal protein sequence data of dihydrolipoamide dehydrogenases from anaerobic, glycine-utilizing bacteria. J Bacteriol. 1990 Apr;172(4):2088–2095. doi: 10.1128/jb.172.4.2088-2095.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg W., Dietrichs D., Lebertz H., Andreesen J. R. Isolation of an atypically small lipoamide dehydrogenase involved in the glycine decarboxylase complex from Eubacterium acidaminophilum. J Bacteriol. 1989 Mar;171(3):1346–1354. doi: 10.1128/jb.171.3.1346-1354.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Jackman S. A., Hough D. W., Danson M. J., Stevenson K. J., Opperdoes F. R. Subcellular localisation of dihydrolipoamide dehydrogenase and detection of lipoic acid in bloodstream forms of Trypanosoma brucei. Eur J Biochem. 1990 Oct 5;193(1):91–95. doi: 10.1111/j.1432-1033.1990.tb19308.x. [DOI] [PubMed] [Google Scholar]
- Kenney W. C., Zakim D., Hogue P. K., Singer T. P. Multiplicity and origin of isoenzymes of lipoyl dehydrogenase. Eur J Biochem. 1972 Jul 13;28(2):253–260. doi: 10.1111/j.1432-1033.1972.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. Pyruvate: ferredoxin oxidoreductase and its activation by ATP in the blue-green alga Anabaena variabilis. Biochim Biophys Acta. 1971 Aug 6;245(1):165–174. doi: 10.1016/0005-2728(71)90019-3. [DOI] [PubMed] [Google Scholar]
- Lohrer H., Krauth-Siegel R. L. Purification and characterization of lipoamide dehydrogenase from Trypanosoma cruzi. Eur J Biochem. 1990 Dec 27;194(3):863–869. doi: 10.1111/j.1432-1033.1990.tb19480.x. [DOI] [PubMed] [Google Scholar]
- MATTHEWS J., REED L. J. Purification and properties of a dihydrolipoic dehydrogenase from Spinacia oleracea. J Biol Chem. 1963 May;238:1869–1876. [PubMed] [Google Scholar]
- Neuer G., Bothe H. The pyruvate: ferredoxin oxidoreductase in heterocysts of the cyanobacterium Anabaena cylindrica. Biochim Biophys Acta. 1982 Jun 16;716(3):358–365. doi: 10.1016/0304-4165(82)90028-9. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt K. J., Carles C., Carne T. J., Danson M. J., Stevenson K. J. Detection of bacterial lipoic acid. A modified gas-chromatographic-mass-spectrometric procedure. Biochem J. 1989 Mar 15;258(3):749–754. doi: 10.1042/bj2580749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D. W., Schulz G. E., Guest J. R. Structural relationship between glutathione reductase and lipoamide dehydrogenase. J Mol Biol. 1984 Apr 15;174(3):483–496. doi: 10.1016/0022-2836(84)90332-2. [DOI] [PubMed] [Google Scholar]
- Richarme G., Heine H. G. Galactose- and maltose-stimulated lipoamide dehydrogenase activities related to the binding-protein-dependent transport of galactose and maltose in toluenized cells of Escherichia coli. Eur J Biochem. 1986 Apr 15;156(2):399–405. doi: 10.1111/j.1432-1033.1986.tb09596.x. [DOI] [PubMed] [Google Scholar]
- Richarme G. Possible involvement of lipoic acid in binding protein-dependent transport systems in Escherichia coli. J Bacteriol. 1985 Apr;162(1):286–293. doi: 10.1128/jb.162.1.286-293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme G. Purification of a new dihydrolipoamide dehydrogenase from Escherichia coli. J Bacteriol. 1989 Dec;171(12):6580–6585. doi: 10.1128/jb.171.12.6580-6585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. R., Reed L. J. Regulation of the activity of the pyruvate dehydrogenase complex of Escherichia coli. Biochemistry. 1970 Mar 17;9(6):1434–1439. doi: 10.1021/bi00808a019. [DOI] [PubMed] [Google Scholar]
- Scouten W. H., McManus I. R. Microbial lipoamide dehydrogenase. Purification and some characteristics of the enzyme derived from selected microorganisms. Biochim Biophys Acta. 1971 Feb 10;227(2):248–263. doi: 10.1016/0005-2744(71)90058-1. [DOI] [PubMed] [Google Scholar]
- Serrano A. Characterization of cyanobacterial ferredoxin-NADP+ oxidoreductase molecular heterogeneity using chromatofocusing. Anal Biochem. 1986 May 1;154(2):441–448. doi: 10.1016/0003-2697(86)90012-6. [DOI] [PubMed] [Google Scholar]
- Serrano A., Losada M. Action spectra for nitrate and nitrite assimilation in blue-green algae. Plant Physiol. 1988 Apr;86(4):1116–1119. doi: 10.1104/pp.86.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A., Rivas J., Losada M. Purification and properties of glutathione reductase from the cyanobacterium Anabaena sp. strain 7119. J Bacteriol. 1984 Apr;158(1):317–324. doi: 10.1128/jb.158.1.317-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Sundquist A. R., Fahey R. C. The novel disulfide reductase bis-gamma-glutamylcystine reductase and dihydrolipoamide dehydrogenase from Halobacterium halobium: purification by immobilized-metal-ion affinity chromatography and properties of the enzymes. J Bacteriol. 1988 Aug;170(8):3459–3467. doi: 10.1128/jb.170.8.3459-3467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J., Strating M. Separation of lipoamide dehydrogenase isoenzymes by affinity chromatography. Biochim Biophys Acta. 1975 Mar 28;384(1):69–80. doi: 10.1016/0005-2744(75)90096-0. [DOI] [PubMed] [Google Scholar]
- Walker G. H., Oliver D. J., Sarojini G. Simultaneous oxidation of glycine and malate by pea leaf mitochondria. Plant Physiol. 1982 Nov;70(5):1465–1469. doi: 10.1104/pp.70.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E. A comparative study of the multiple forms of pig heart lipoyl dehydrogenase. Arch Biochem Biophys. 1971 May;144(1):216–223. doi: 10.1016/0003-9861(71)90471-1. [DOI] [PubMed] [Google Scholar]
- Yanagawa H., Egami F. Asparagusate dehydrogenases and lipoyl dehydrogenase from asparagus mitochondria. Physical, chemical, and enzymatic properties. J Biol Chem. 1976 Jun 25;251(12):3637–3644. [PubMed] [Google Scholar]
- Yang V. C., Langer R. pH-dependent binding analysis, a new and rapid method for isoelectric point estimation. Anal Biochem. 1985 May 15;147(1):148–155. doi: 10.1016/0003-2697(85)90021-1. [DOI] [PubMed] [Google Scholar]
- Yocum R. R., Blumberg P. M., Strominger J. L. Purification and characterization of the thermophilic D-alanine carboxypeptidase from membranes of Bacillus stearothermophilus. J Biol Chem. 1974 Aug 10;249(15):4863–4871. [PubMed] [Google Scholar]
- Zimmer G., Mainka L., Krüger E. Dihydrolipoic acid activates oligomycin-sensitive thiol groups and increases ATP synthesis in mitochondria. Arch Biochem Biophys. 1991 Aug 1;288(2):609–613. doi: 10.1016/0003-9861(91)90243-c. [DOI] [PubMed] [Google Scholar]