Abstract
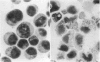
Treatment of HL-60 cells with 0.5 mM-butyric acid resulted in morphological changes, including the formation of cytoplasmic granules, nuclear condensation and segmentation. These differentiated cells had an elevated phospholipase A2 activity and an increased capacity to synthesize a variety of eicosanoids, including both lipoxygenase and cyclooxygenase products. Phospholipase A2-mediated release of arachidonic acid is accompanied by an equimolar production of potentially cytotoxic lysophospholipid. In association with the differentiation process, there was a 2-3-fold increase in lysophospholipase activity. Subsequent studies were undertaken to identify and characterize the lysophospholipases in this cell system, with 1-[1-14C]palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine as substrate. Hydrophobic chromatography of both undifferentiated and differentiated cell extracts revealed three peaks of enzyme activity. Extracts of differentiated cells contained a dramatic increase in activity contained in peak 2. The increase in enzymic activity of peak 2 appeared to account for the increase in total lysophospholipase activity found in the differentiated cell homogenates. The lysophospholipases contained in peaks 2 and 3 were purified to homogeneity and were 20 and 22 kDa respectively, as determined by denaturing polyacrylamide-gel electrophoresis. Peaks 2 and 3 were similar on the basis of amino acid composition, but had distinctive C-terminal peptide amino acid sequences. Enzymic characterization of these proteins demonstrated that there was no detectable level of non-specific esterase, acyltransferase or transacylase activity associated with these proteins. We concluded that peak 2 lysophospholipase is regulated by differentiation in HL-60 cells and may play an important role in protecting these cells from the cytolytic effects of the lysophospholipids produced by the activation of phospholipase A2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agins A. P., Zipkin R. E., Taffer I. M. Metabolism of cyclooxygenase and lipoxygenase products by 15-prostaglandin dehydrogenase from human HL-60 leukemia cells. Agents Actions. 1987 Aug;21(3-4):397–399. doi: 10.1007/BF01966527. [DOI] [PubMed] [Google Scholar]
- Anthes J. C., Bryant R. W., Musch M. W., Ng K., Siegel M. I. Calcium ionophore and chemotactic peptide stimulation of peptidoleukotriene synthesis in DMSO-differentiated HL60 cells. Inflammation. 1986 Jun;10(2):145–156. doi: 10.1007/BF00915996. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Milona N., Knopf J. L. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Conway T. M., Mong S., Steiner S., Crooke S. T. Leukotriene D4 treatment of bovine aortic endothelial cells and murine smooth muscle cells in culture results in an increase in phospholipase A2 activity. J Biol Chem. 1986 Aug 15;261(23):10713–10718. [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., van den Berg J. W., van den Bosch H., van Deenen L. L. Metabolism of phospholipids by polymorphonuclear leukocytes. Biochim Biophys Acta. 1965 Oct 4;106(2):338–347. doi: 10.1016/0005-2760(65)90042-1. [DOI] [PubMed] [Google Scholar]
- Fischkoff S. A., Brown G. E., Pollak A. Synthesis of eosinophil-associated enzymes in HL-60 promyelocytic leukemia cells. Blood. 1986 Jul;68(1):185–192. [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. The importance of phospholipase-A2 in prostaglandin biosynthesis. Biochem Pharmacol. 1976 Feb 1;25(3):285–291. doi: 10.1016/0006-2952(76)90216-1. [DOI] [PubMed] [Google Scholar]
- Goerig M., Habenicht A. J., Zeh W., Salbach P., Kommerell B., Rothe D. E., Nastainczyk W., Glomset J. A. Evidence for coordinate, selective regulation of eicosanoid synthesis in platelet-derived growth factor-stimulated 3T3 fibroblasts and in HL-60 cells induced to differentiate into macrophages or neutrophils. J Biol Chem. 1988 Dec 25;263(36):19384–19391. [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. W., Drisdel R. C., Sobel B. E. Rabbit myocardial lysophospholipase-transacylase. Purification, characterization, and inhibition by endogenous cardiac amphiphiles. J Biol Chem. 1983 Dec 25;258(24):15165–15172. [PubMed] [Google Scholar]
- Gross R. W., Sobel B. E. Lysophosphatidylcholine metabolism in the rabbit heart. Characterization of metabolic pathways and partial purification of myocardial lysophospholipase-transacylase. J Biol Chem. 1982 Jun 25;257(12):6702–6708. [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hoessly M. C., Rossi R. M., Fischkoff S. A. Factors responsible for variable reported lineages of HL-60 cells induced to mature with butyric acid. Cancer Res. 1989 Jul 1;49(13):3594–3597. [PubMed] [Google Scholar]
- Kramer R. M., Roberts E. F., Manetta J., Putnam J. E. The Ca2(+)-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J Biol Chem. 1991 Mar 15;266(8):5268–5272. [PubMed] [Google Scholar]
- Kugiyama K., Kerns S. A., Morrisett J. D., Roberts R., Henry P. D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990 Mar 8;344(6262):160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- Leslie C. C. Kinetic properties of a high molecular mass arachidonoyl-hydrolyzing phospholipase A2 that exhibits lysophospholipase activity. J Biol Chem. 1991 Jun 15;266(17):11366–11371. [PubMed] [Google Scholar]
- Oishi K., Raynor R. L., Charp P. A., Kuo J. F. Regulation of protein kinase C by lysophospholipids. Potential role in signal transduction. J Biol Chem. 1988 May 15;263(14):6865–6871. [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Moritz R. L., Begg G. S., Rubira M. R., Nice E. C. Micropreparative procedures for high sensitivity sequencing of peptides and proteins. Anal Biochem. 1989 Mar;177(2):221–236. doi: 10.1016/0003-2697(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Bach D. S., Austen K. F. Biochemical characterization of human eosinophil Charcot-Leyden crystal protein (lysophospholipase). J Biol Chem. 1984 Dec 25;259(24):15100–15105. [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y., Deems R. A., Dennis E. A. Lysophospholipases I and II from P388D1 macrophage-like cell line. Methods Enzymol. 1991;197:456–468. doi: 10.1016/0076-6879(91)97171-t. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y., Dennis E. A. Purification and characterization of a lysophospholipase from a macrophage-like cell line P388D1. J Biol Chem. 1988 Jul 15;263(20):9965–9972. [PubMed] [Google Scholar]
- Ziboh V. A., Wong T., Wu M. C., Yunis A. A. Lipoxygenation of arachidonic acid by differentiated and undifferentiated human promyelocytic HL-60 cells. J Lab Clin Med. 1986 Aug;108(2):161–166. [PubMed] [Google Scholar]
- de Jong J. G., van den Bosch H., Aarsman A. J., van Deenen L. L. Studies on lysophospholipases. II. Substrate specificity of a lysolecithin hydrolyzing carboxylesterase from beef pancreas. Biochim Biophys Acta. 1973 Jan 19;296(1):105–115. doi: 10.1016/0005-2760(73)90049-0. [DOI] [PubMed] [Google Scholar]
- de Jong J. G., van den Bosch H., Rijken D., van Deenen L. L. Studies on lysophospholipases. 3. The complete purification of two proteins with lysophospholipase activity from beef liver. Biochim Biophys Acta. 1974 Oct 16;369(1):50–63. doi: 10.1016/0005-2760(74)90191-x. [DOI] [PubMed] [Google Scholar]