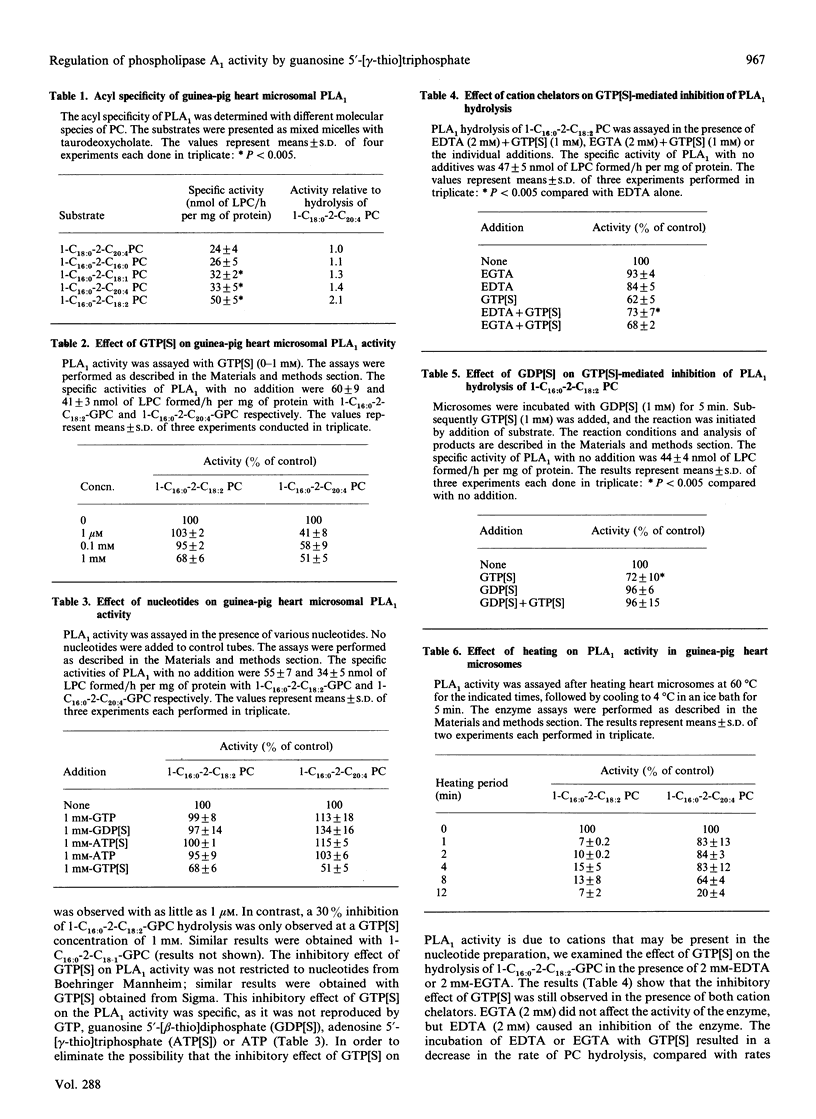
Abstract
We have recently characterized lysophospholipase A2 activities in guinea-pig heart microsomes and postulated that these enzymes act sequentially with phospholipases A1 to release fatty acids selectively from phosphatidylcholine (PC) and phosphatidylethanolamine, thus providing an alternative route to the phospholipase A2 mode of release. In a further investigation of the postulated pathway, we have characterized the PC-hydrolysing phospholipase A1 in guinea-pig heart microsomes. Our results show that the enzyme may have a preference for substrates with C16:0 over C18:0 at the sn-1 position. In addition, although the enzyme cleaves the sn-1 fatty acid, the rate of hydrolysis of PC substrates with C16:0 at the sn-1 position was influenced by the nature of the fatty acid at the sn-2 position. The order of decreasing preference was C18:2 > C20:4 = C18:1 > C16:0. The hydrolyses of the molecular species were differentially affected by heating at 60 degrees C. An investigation into the effect of nucleotides on the activity of the enzyme showed that guanosine 5'-[gamma-thio]triphosphate (GTP[S]) inhibited the hydrolysis of PC by phospholipase A1 activity, whereas GTP, guanosine 5'-[beta-thio]diphosphate (GDP[S]), GDP, ATP and adenosine 5'-[gamma-thio]triphosphate (ATP[S]) did not affect the activity. The inhibitory effect of GTP[S] on phospholipase A1 activity was blocked by preincubation with GDP[S]. A differential effect of GTP[S] on the hydrolysis of different molecular species was also observed. Taken together, the results of this study suggest the presence of more than one phospholipase A1 in the microsomes with different substrate specificities, which act sequentially with lysophospholipase A2 to release linoleic or arachidonic acid selectively from PC under resting conditions. Upon stimulation and activation of the G-protein, the release of fatty acids would be inhibited.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur G. Lysophospholipase A2 activity in guinea-pig heart microsomal fractions displaying high activities with 2-acylglycerophosphocholines with linoleic and arachidonic acids. Biochem J. 1989 Jul 15;261(2):581–586. doi: 10.1042/bj2610581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur G., Mock T., Zaborniak C., Choy P. C. The distribution and acyl composition of plasmalogens in guinea pig heart. Lipids. 1985 Oct;20(10):693–698. doi: 10.1007/BF02534389. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem Soc Trans. 1990 Aug;18(4):503–507. doi: 10.1042/bst0180503. [DOI] [PubMed] [Google Scholar]
- Badiani K., Arthur G. 2-acyl-sn-glycero-3-phosphoethanolamine lysophospholipase A2 activity in guinea-pig heart microsomes. Biochem J. 1991 Apr 15;275(Pt 2):393–398. doi: 10.1042/bj2750393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C., Mullmann T. J. Receptor-coupled phospholipase D: regulation and functional significance. Biochem Soc Trans. 1991 Apr;19(2):324–329. doi: 10.1042/bst0190324. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Buchanan M. R., Bastida E. Endothelium and underlying membrane reactivity with platelets, leukocytes and tumor cells: regulation by the lipoxygenase-derived fatty acid metabolites, 13-HODE and HETES. Med Hypotheses. 1988 Dec;27(4):317–325. doi: 10.1016/0306-9877(88)90014-x. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. The control of free arachidonic acid levels. Trends Biochem Sci. 1990 Oct;15(10):365–366. doi: 10.1016/0968-0004(90)90227-3. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Tam S. W., Arthur G., Chen H., Choy P. C. The purification and characterization of a phospholipase A in hamster heart cytosol for the hydrolysis of phosphatidylcholine. J Biol Chem. 1987 Dec 15;262(35):16927–16935. [PubMed] [Google Scholar]
- Cockcroft S., Nielson C. P., Stutchfield J. Is phospholipase A2 activation regulated by G-proteins? Biochem Soc Trans. 1991 Apr;19(2):333–336. doi: 10.1042/bst0190333. [DOI] [PubMed] [Google Scholar]
- Haas T. A., Bertomeu M. C., Bastida E., Buchanan M. R. Cyclic AMP regulation of endothelial cell triacylglycerol turnover, 13-hydroxyoctadecadienoic acid (13-HODE) synthesis and endothelial cell thrombogenicity. Biochim Biophys Acta. 1990 Feb 19;1051(2):174–178. doi: 10.1016/0167-4889(90)90190-o. [DOI] [PubMed] [Google Scholar]
- Harden T. K., Stephens L., Hawkins P. T., Downes C. P. Turkey erythrocyte membranes as a model for regulation of phospholipase C by guanine nucleotides. J Biol Chem. 1987 Jul 5;262(19):9057–9061. [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsema C. L. Light activation of phospholipase A2 in rod outer segments of bovine retina and its modulation by GTP-binding proteins. J Biol Chem. 1987 Jan 5;262(1):163–168. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Schalkwijk C. G., Märki F., Van den Bosch H. Studies on the acyl-chain selectivity of cellular phospholipases A2. Biochim Biophys Acta. 1990 May 1;1044(1):139–146. doi: 10.1016/0005-2760(90)90229-q. [DOI] [PubMed] [Google Scholar]
- Silk S. T., Clejan S., Witkom K. Evidence of GTP-binding protein regulation of phospholipase A2 activity in isolated human platelet membranes. J Biol Chem. 1989 Dec 25;264(36):21466–21469. [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Trotz M., Hein L., Hostetler K. Y. Solubilization and partial characterization of phospholipase A from rat heart sarcoplasmic reticulum. Biochim Biophys Acta. 1988 Sep 23;962(2):248–257. doi: 10.1016/0005-2760(88)90167-1. [DOI] [PubMed] [Google Scholar]
- van den Bosch H., Aarsman A. J., van Schaik R. H., Schalkwijk C. G., Neijs F. W., Sturk A. Structural and enzymological properties of cellular phospholipases A2. Biochem Soc Trans. 1990 Oct;18(5):781–785. doi: 10.1042/bst0180781. [DOI] [PubMed] [Google Scholar]


