Abstract
The substrate-induced inactivation of beta-lactamase I from Bacillus cereus 569/H has been studied. Both the wild-type enzyme and mutants have been used. The kinetics follow a branched pathway of the type recently analysed [Waley (1991) Biochem. J. 279, 87-94]. The substrate cloxacillin (a penicillin) formed an acyl-enzyme (characterized by m.s.), and it was probably the instability of this intermediate that brought about inactivation. A disulphide bond was introduced into beta-lactamase I (the wild-type enzyme lacks this bond) by site-directed mutagenesis: Ala-77 and Ala-123 were replaced by cysteine. Spontaneous oxidation yielded the disulphide. The activity of this newly cross-linked enzyme was a little diminished, but the stability towards inactivation by cloxacillin was not increased. A second mutant of beta-lactamase I was studied: this mutant lacked the first 17 residues, i.e. the first alpha-helix. The mutant had reduced activity towards ordinary (non-inactivating) substrates and no hydrolysis of cloxacillin could be detected. These mutant enzymes were expressed in Bacillus subtilis, and were purified from the extracellular medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Coulson A. F., Frère J. M., Ghuysen J. M., Joris B., Forsman M., Levesque R. C., Tiraby G., Waley S. G. A standard numbering scheme for the class A beta-lactamases. Biochem J. 1991 May 15;276(Pt 1):269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin R. T., Baldwin J. E., Schofield C. J., Waley S. G. Use of electrospray mass spectrometry to directly observe an acyl enzyme intermediate in beta-lactamase catalysis. FEBS Lett. 1990 Dec 17;277(1-2):212–214. doi: 10.1016/0014-5793(90)80847-c. [DOI] [PubMed] [Google Scholar]
- Blanpain P. C., Nagy J. B., Laurent G. H., Durant F. V. A multifaceted approach to the study of the side-chain conformation in beta-lactamase-resistant penicillins. J Med Chem. 1980 Dec;23(12):1283–1292. doi: 10.1021/jm00186a002. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- CROMPTON B., JAGO M., CRAWFORD K., NEWTON G. G., ABRAHAM E. P. Behaviour of some derivatives of 7-aminocephalosporanic acid and 6-aminopenicillanic acidas substrates, inhibitors and inducers of penicillinases. Biochem J. 1962 Apr;83:52–63. doi: 10.1042/bj0830052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey E. A., Pain R. H. Conformation of a stable intermediate on the folding pathway of Staphylococcus aureus penicillinase. Biochim Biophys Acta. 1978 Mar 28;533(1):12–22. doi: 10.1016/0005-2795(78)90542-1. [DOI] [PubMed] [Google Scholar]
- Christensen H., Martin M. T., Waley S. G. Beta-lactamases as fully efficient enzymes. Determination of all the rate constants in the acyl-enzyme mechanism. Biochem J. 1990 Mar 15;266(3):853–861. [PMC free article] [PubMed] [Google Scholar]
- Christensen H., Pain R. H. Molten globule intermediates and protein folding. Eur Biophys J. 1991;19(5):221–229. doi: 10.1007/BF00183530. [DOI] [PubMed] [Google Scholar]
- Citri N., Samuni A., Zyk N. Acquisition of substrate-specific parameters during the catalytic reaction of penicillinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1048–1052. doi: 10.1073/pnas.73.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G. Regression analysis of nonlinear Arrhenius plots: an empirical model and a computer program. Comput Biol Med. 1984;14(4):447–455. doi: 10.1016/0010-4825(84)90045-3. [DOI] [PubMed] [Google Scholar]
- Fink A. L., Behner K. M., Tan A. K. Kinetic and structural characterization of reversibly inactivated beta-lactamase. Biochemistry. 1987 Jul 14;26(14):4248–4258. doi: 10.1021/bi00388a011. [DOI] [PubMed] [Google Scholar]
- Frère J. M. Interaction between serine beta-lactamases and class A substrates: a kinetic analysis and a reaction pathway hypothesis. Biochem Pharmacol. 1981 Mar 15;30(6):549–552. doi: 10.1016/0006-2952(81)90124-6. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B., Granier B., Matagne A., Jacob F., Bourguignon-Bellefroid C. Diversity of the mechanisms of resistance to beta-lactam antibiotics. Res Microbiol. 1991 Jul-Aug;142(6):705–710. doi: 10.1016/0923-2508(91)90084-n. [DOI] [PubMed] [Google Scholar]
- Gibson R. M., Christensen H., Waley S. G. Site-directed mutagenesis of beta-lactamase I. Single and double mutants of Glu-166 and Lys-73. Biochem J. 1990 Dec 15;272(3):613–619. doi: 10.1042/bj2720613. [DOI] [PMC free article] [PubMed] [Google Scholar]
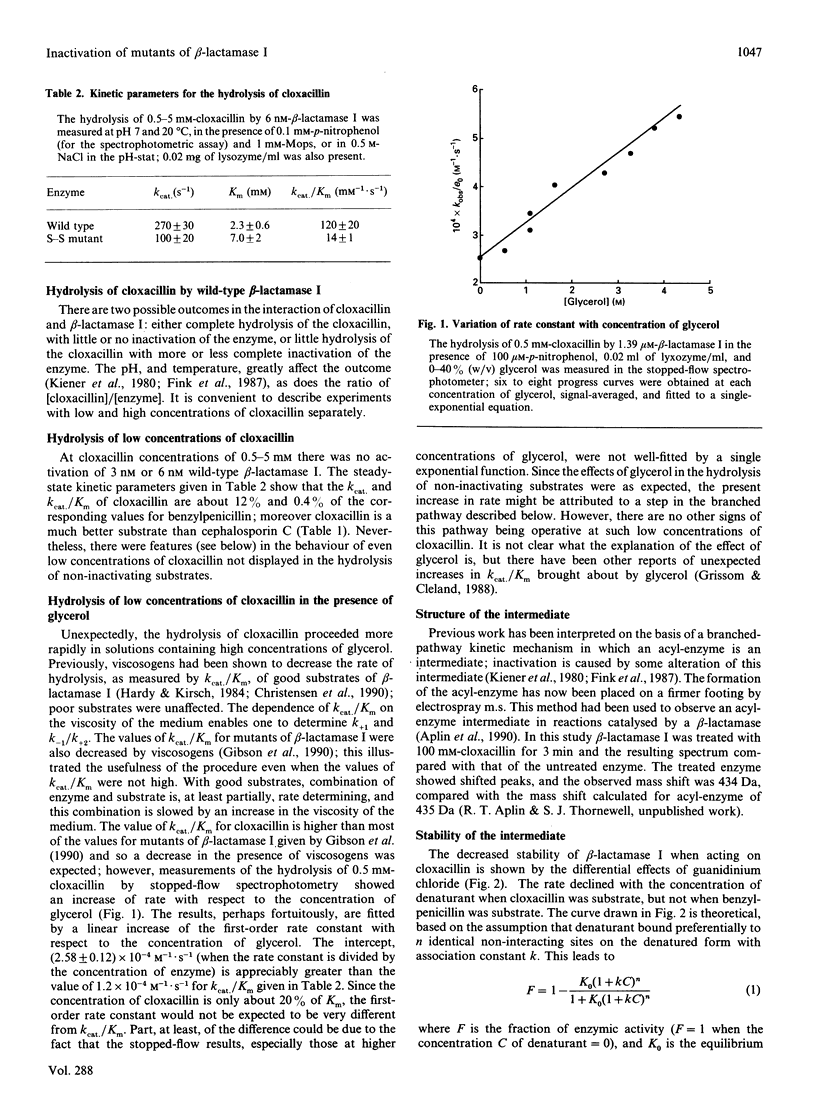
- Grissom C. B., Cleland W. W. Isotope effect studies of chicken liver NADP malic enzyme: role of the metal ion and viscosity dependence. Biochemistry. 1988 Apr 19;27(8):2927–2934. doi: 10.1021/bi00408a039. [DOI] [PubMed] [Google Scholar]
- Hardy L. W., Kirsch J. F. Diffusion-limited component of reactions catalyzed by Bacillus cereus beta-lactamase I. Biochemistry. 1984 Mar;23(6):1275–1282. doi: 10.1021/bi00301a040. [DOI] [PubMed] [Google Scholar]
- Herzberg O. Refined crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.0 A resolution. J Mol Biol. 1991 Feb 20;217(4):701–719. doi: 10.1016/0022-2836(91)90527-d. [DOI] [PubMed] [Google Scholar]
- Jelsch C., Lenfant F., Masson J. M., Samama J. P. Beta-lactamase TEM1 of E. coli. Crystal structure determination at 2.5 A resolution. FEBS Lett. 1992 Mar 9;299(2):135–142. doi: 10.1016/0014-5793(92)80232-6. [DOI] [PubMed] [Google Scholar]
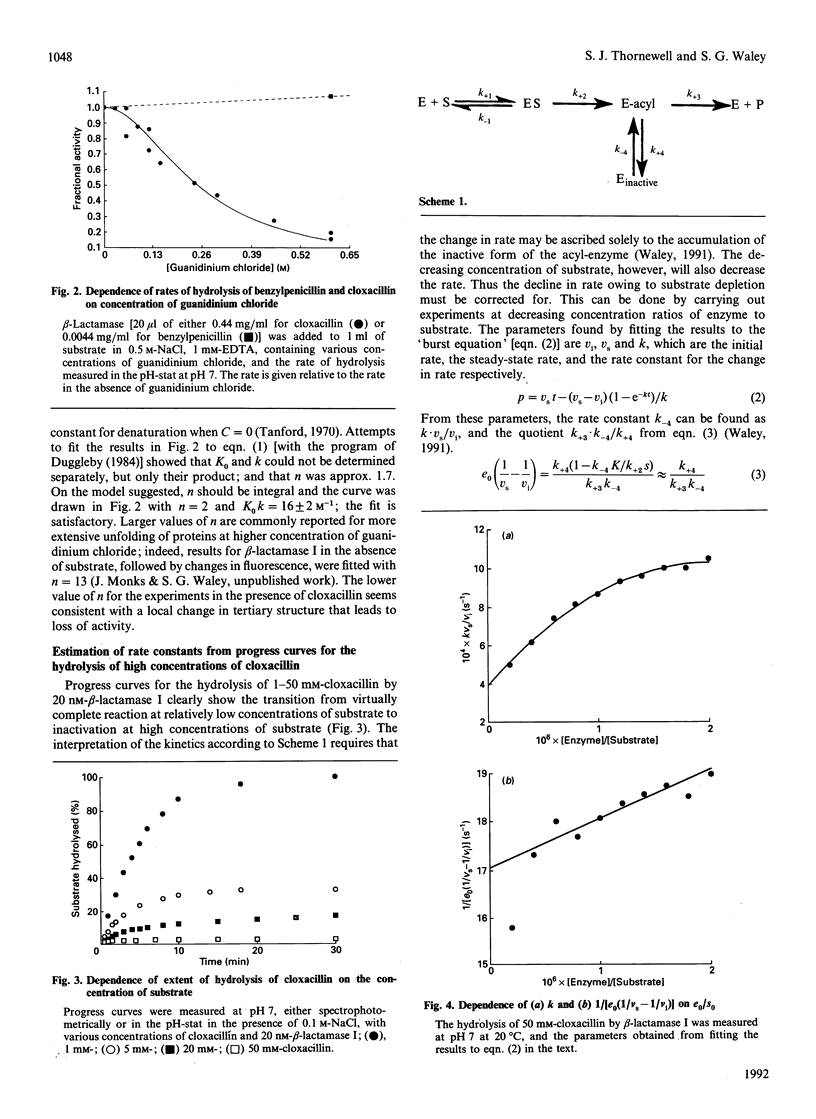
- Kiener P. A., Knott-Hunziker V., Petursson S., Waley S. G. Mechanism of substrate-induced inactivation of beta-lactamase I. Eur J Biochem. 1980 Aug;109(2):575–580. doi: 10.1111/j.1432-1033.1980.tb04830.x. [DOI] [PubMed] [Google Scholar]
- Kiener P. A., Waley S. G. Substrate-induced deactivation of penicillinases. Studies of beta-lactamase I by hydrogen exchange. Biochem J. 1977 Aug 1;165(2):279–285. doi: 10.1042/bj1650279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J. R., Moews P. C. Beta-lactamase of Bacillus licheniformis 749/C. Refinement at 2 A resolution and analysis of hydration. J Mol Biol. 1991 Jul 20;220(2):435–455. doi: 10.1016/0022-2836(91)90023-y. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Matagne A., Joris B., Van Beeumen J., Frère J. M. Ragged N-termini and other variants of class A beta-lactamases analysed by chromatofocusing. Biochem J. 1991 Feb 1;273(Pt 3):503–510. doi: 10.1042/bj2730503. [DOI] [PMC free article] [PubMed] [Google Scholar]
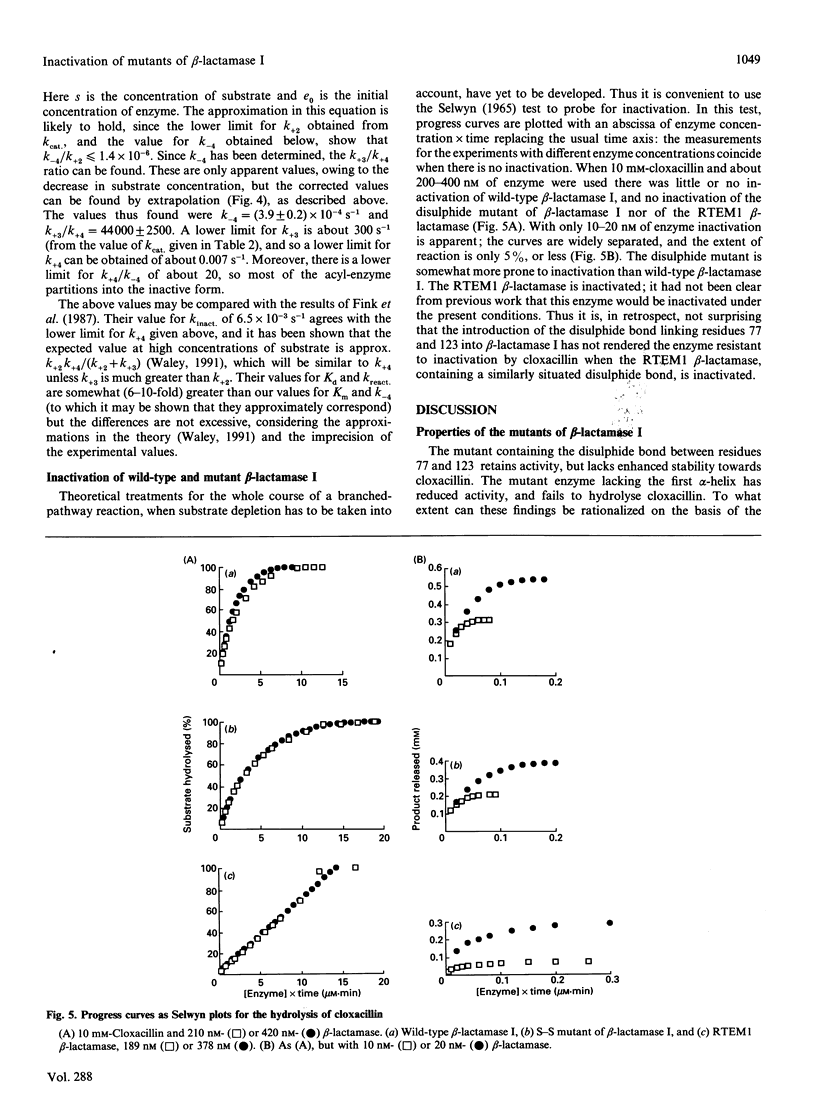
- Matagne A., Misselyn-Bauduin A. M., Joris B., Erpicum T., Granier B., Frère J. M. The diversity of the catalytic properties of class A beta-lactamases. Biochem J. 1990 Jan 1;265(1):131–146. doi: 10.1042/bj2650131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. STIMULATING AND INHIBITING ANTIBODIES FOR BACTERIAL PENICILLINASE. Immunology. 1964 Nov;7:707–723. [PMC free article] [PubMed] [Google Scholar]
- Persaud K. C., Pain R. H., Virden R. Reversible deactivation of beta-lactamase by quinacillin. Extent of the conformational change in the isolated transitory complex. Biochem J. 1986 Aug 1;237(3):723–730. doi: 10.1042/bj2370723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Samraoui B., Sutton B. J., Todd R. J., Artymiuk P. J., Waley S. G., Phillips D. C. Tertiary structural similarity between a class A beta-lactamase and a penicillin-sensitive D-alanyl carboxypeptidase-transpeptidase. 1986 Mar 27-Apr 2Nature. 320(6060):378–380. doi: 10.1038/320378a0. [DOI] [PubMed] [Google Scholar]
- Samuni A., Citri N. How specific is the effect of penicillins on the conformation of penicillinase? An experimental model. Mol Pharmacol. 1979 Jul;16(1):250–255. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn M. J. A simple test for inactivation of an enzyme during assay. Biochim Biophys Acta. 1965 Jul 29;105(1):193–195. doi: 10.1016/s0926-6593(65)80190-4. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- Waley S. G. Beta-lactamases: a major cause of antibiotic resistance. Sci Prog. 1988;72(288 Pt 4):579–597. [PubMed] [Google Scholar]
- Waley S. G. Kinetic parameters from progress curves of competing substrates. Application to beta-lactamases. Biochem J. 1983 May 1;211(2):511–513. doi: 10.1042/bj2110511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. The kinetics of substrate-induced inactivation. Biochem J. 1991 Oct 1;279(Pt 1):87–94. doi: 10.1042/bj2790087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton C. W., Szawelski R. J. Half-time analysis of the integrated Michaelis equation. Simulation and use of the half-time plot and its direct linear variant in the analysis of some alpha-chymotrypsin, papain- and fumarase-catalysed reactions. Biochem J. 1982 May 1;203(2):351–360. doi: 10.1042/bj2030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyk N., Citri N. The interaction of penicillinase with penicillins. V. Conformative response constants. Biochim Biophys Acta. 1967 Sep 12;146(1):219–226. doi: 10.1016/0005-2744(67)90088-5. [DOI] [PubMed] [Google Scholar]
- Zyk N., Citri N. The interaction of penicillinase with penicillins. VI. Comparison of free and antibody-bound enzyme. Biochim Biophys Acta. 1968 Jun 4;159(2):317–326. doi: 10.1016/0005-2744(68)90080-6. [DOI] [PubMed] [Google Scholar]
- Zyk N., Citri N. The interaction of penicillinase with penicillins. VII. Effect of specific antibodies on conformative response. Biochim Biophys Acta. 1968 Jun 4;159(2):327–339. doi: 10.1016/0005-2744(68)90081-8. [DOI] [PubMed] [Google Scholar]


