Abstract
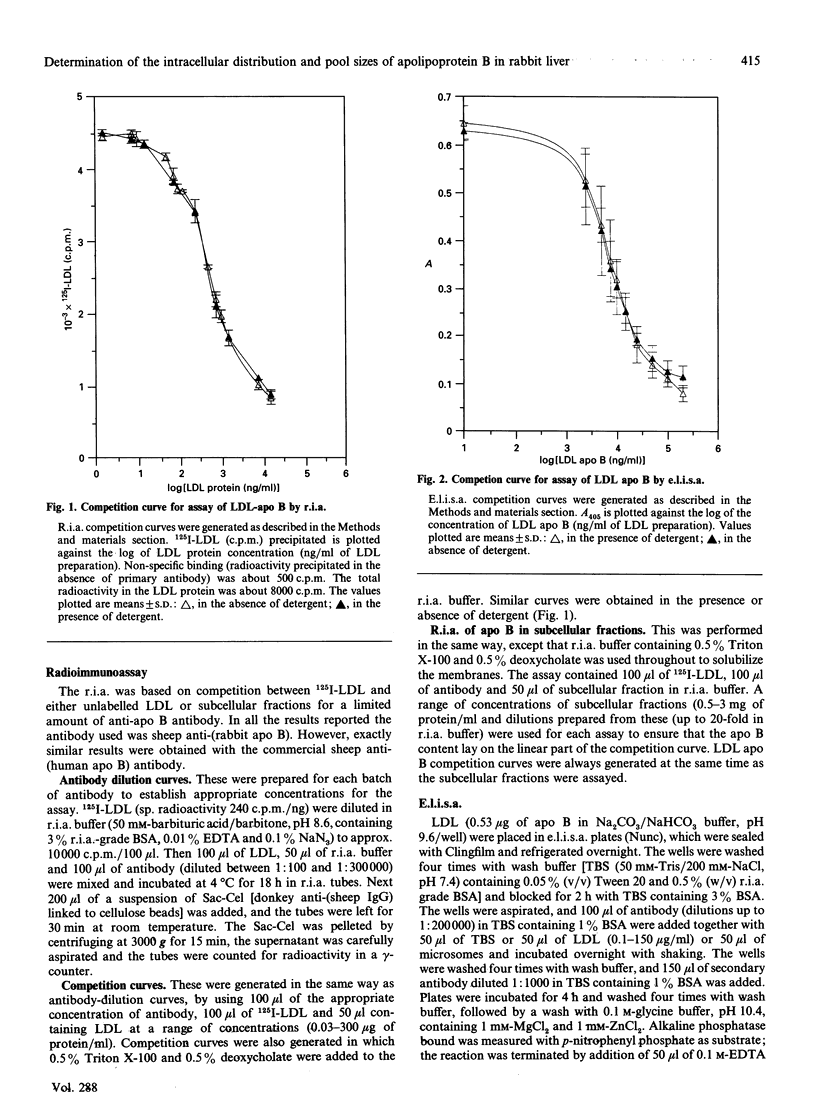
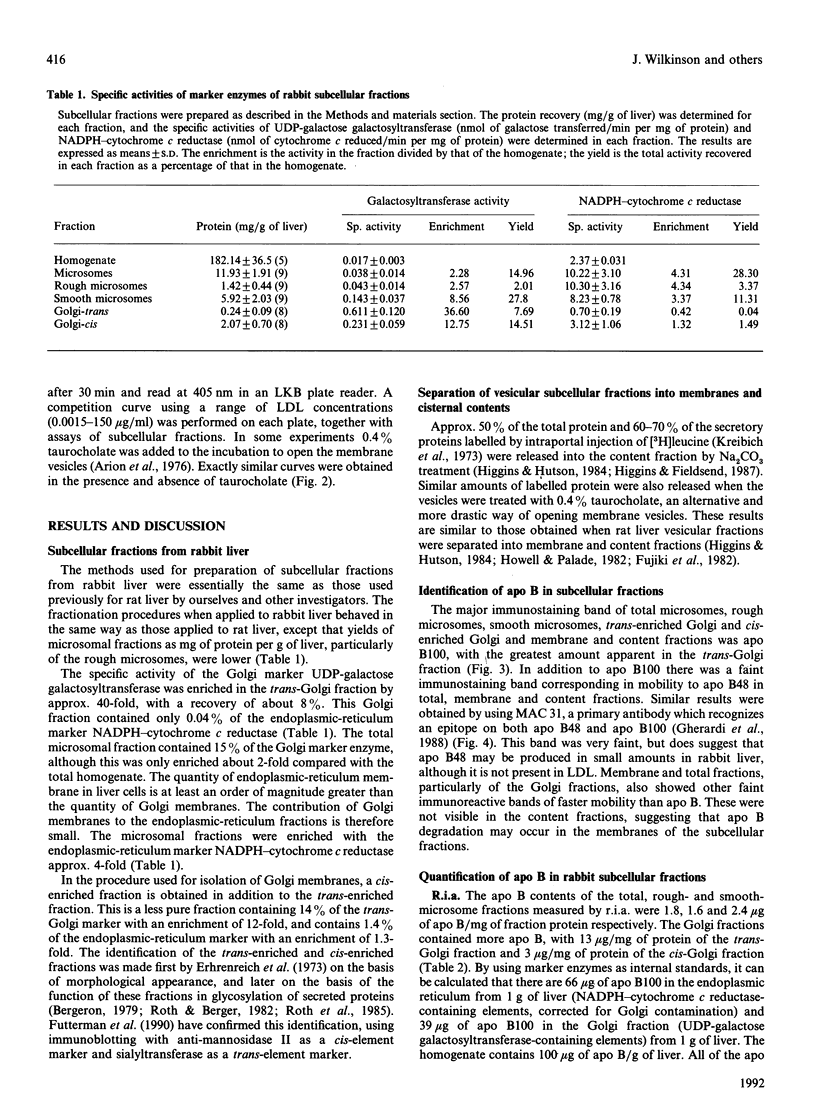
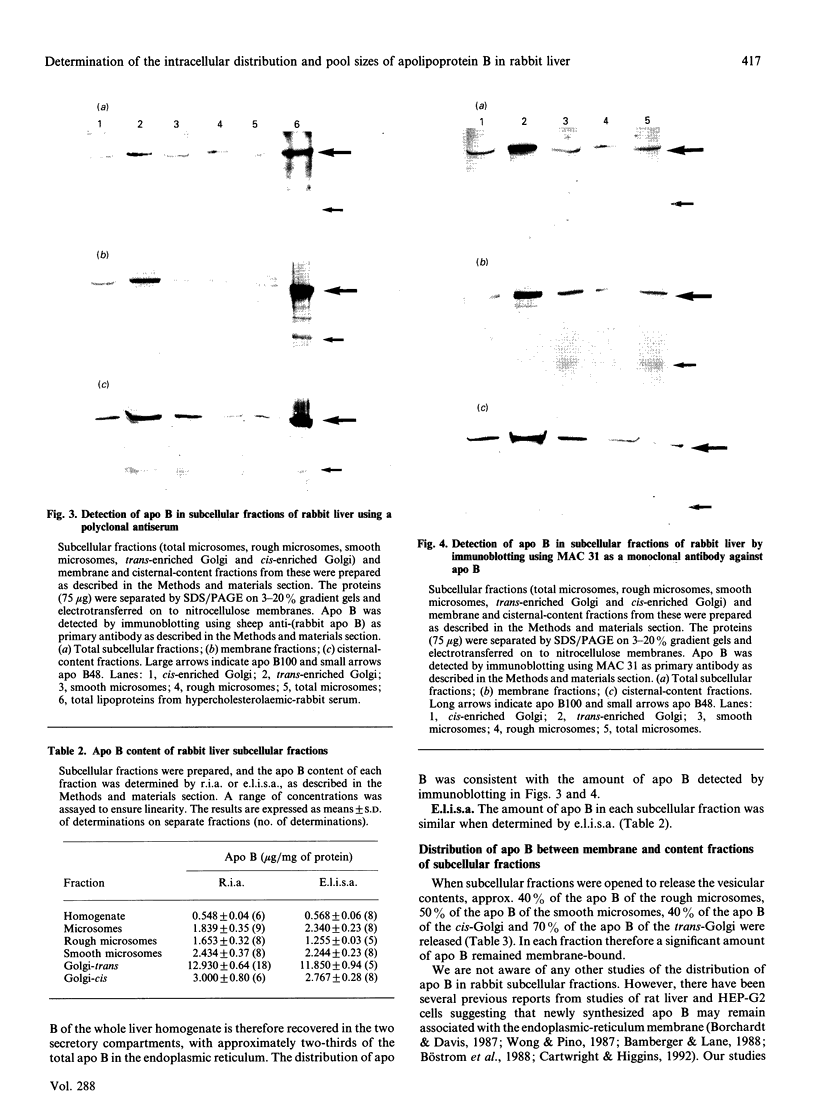
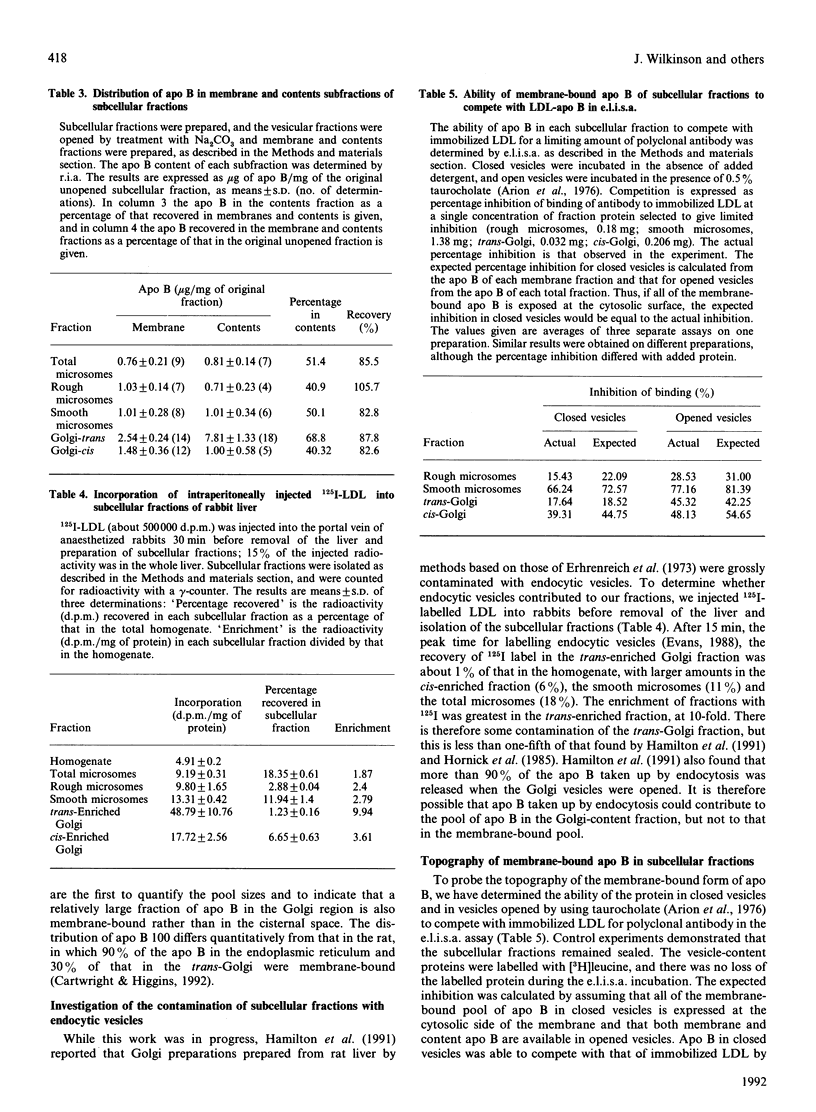
We have investigated the intracellular distribution of apolipoprotein B (apo B) in rabbit liver by immunoblotting, radioimmunoassay (r.i.a.) and enzyme-linked immunoassay (e.l.i.s.a.). Apo B100 was detected in total microsomes, rough microsomes, smooth microsomes, trans-enriched Golgi and cis-enriched Golgi and membrane and cisternal-content subfractions prepared from these fractions. There was also evidence of degradation of apo B100 in the Golgi membrane fractions. The amount of apo B in the subcellular fractions detected by competitive r.i.a. or e.l.i.s.a. ranged from 1.5 micrograms/mg of protein in the rough endoplasmic reticulum to 13 micrograms/mg of protein in the trans-Golgi fraction. Using internal standards (NADPH-cytochrome c reductase for the endoplasmic reticulum and galactosyltransferase for the Golgi membranes) it was calculated that all the apo B of liver is recovered within the secretory compartment, with 63% of the total apo B in the endoplasmic reticulum and the remainder in the Golgi. When the subcellular fractions were separated into membranes and cisternal contents, 60%, 50%, 60% and 30% of the total apo B was recovered in the membrane of the rough microsomes, smooth microsomes, cis-Golgi and trans-Golgi respectively. Using competitive e.l.i.s.a. we found that the membrane-bound form of the apo B was exposed at the cytosolic surface of the intact subcellular fractions. These observations are consistent with a model for assembly of very-low-density lipoproteins (VLDL) in which newly synthesized apo B is incorporated into a membrane-bound pool and a lumenal pool. The membrane-bound pool not used for VLDL assembly may be degraded, possibly in the Golgi region.
Full text
PDF


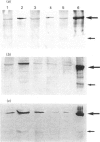
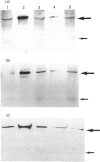
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger M. J., Lane M. D. Assembly of very low density lipoprotein in the hepatocyte. Differential transport of apoproteins through the secretory pathway. J Biol Chem. 1988 Aug 25;263(24):11868–11878. [PubMed] [Google Scholar]
- Bergeron J. J. Golgi fractions from livers of control and ethanol-intoxicated rats. Enzymic and morphologic properties following rapid isolation. Biochim Biophys Acta. 1979 Aug 23;555(3):493–503. doi: 10.1016/0005-2736(79)90402-4. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Borchardt R. A., Davis R. A. Intrahepatic assembly of very low density lipoproteins. Rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987 Dec 5;262(34):16394–16402. [PubMed] [Google Scholar]
- Boström K., Borén J., Wettesten M., Sjöberg A., Bondjers G., Wiklund O., Carlsson P., Olofsson S. O. Studies on the assembly of apo B-100-containing lipoproteins in HepG2 cells. J Biol Chem. 1988 Mar 25;263(9):4434–4442. [PubMed] [Google Scholar]
- Bussmann L. E., Ward S., Kuhn N. J. Lactose and fatty acid synthesis in lactating-rat mammary gland. Effects of starvation, re-feeding, and administration of insulin, adrenaline, streptozotocin and 2-bromo-alpha-ergocryptine. Biochem J. 1984 Apr 1;219(1):173–180. doi: 10.1042/bj2190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright I. J., Higgins J. A. Quantification of apolipoprotein B-48 and B-100 in rat liver endoplasmic reticulum and Golgi fractions. Biochem J. 1992 Jul 1;285(Pt 1):153–159. doi: 10.1042/bj2850153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck S. L., Yao Z., Blackhart B. D., McCarthy B. J., Lingappa V. R. New variation on the translocation of proteins during early biogenesis of apolipoprotein B. Nature. 1990 Jul 26;346(6282):382–385. doi: 10.1038/346382a0. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Thrift R. N., Wu C. C., Howell K. E. Apolipoprotein B is both integrated into and translocated across the endoplasmic reticulum membrane. Evidence for two functionally distinct pools. J Biol Chem. 1990 Jun 15;265(17):10005–10011. [PubMed] [Google Scholar]
- Dixon J. L., Furukawa S., Ginsberg H. N. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem. 1991 Mar 15;266(8):5080–5086. [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Stieger B., Hubbard A. L., Pagano R. E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990 May 25;265(15):8650–8657. [PubMed] [Google Scholar]
- Gherardi E., Hutchings A., Galfre G., Bowyer D. E. Rat monoclonal antibodies to rabbit and human serum low-density lipoprotein. Biochem J. 1988 May 15;252(1):237–245. doi: 10.1042/bj2520237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Moorehouse A., Havel R. J. Isolation and properties of nascent lipoproteins from highly purified rat hepatocytic Golgi fractions. J Lipid Res. 1991 Mar;32(3):529–543. [PubMed] [Google Scholar]
- Higgins J. A. Evidence that during very low density lipoprotein assembly in rat hepatocytes most of the triacylglycerol and phospholipid are packaged with apolipoprotein B in the Golgi complex. FEBS Lett. 1988 May 23;232(2):405–408. doi: 10.1016/0014-5793(88)80780-4. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Fieldsend J. K. Phosphatidylcholine synthesis for incorporation into membranes or for secretion as plasma lipoproteins by Golgi membranes of rat liver. J Lipid Res. 1987 Mar;28(3):268–278. [PubMed] [Google Scholar]
- Higgins J. A. Heterogeneity of phospholipid synthesis in rat liver endoplasmic reticulum during proliferation of smooth membranes. J Cell Sci. 1976 Oct;22(1):173–197. doi: 10.1242/jcs.22.1.173. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Hutson J. L. The roles of Golgi and endoplasmic reticulum in the synthesis and assembly of lipoprotein lipids in rat hepatocytes. J Lipid Res. 1984 Dec 1;25(12):1295–1305. [PubMed] [Google Scholar]
- Hornick C. A., Hamilton R. L., Spaziani E., Enders G. H., Havel R. J. Isolation and characterization of multivesicular bodies from rat hepatocytes: an organelle distinct from secretory vesicles of the Golgi apparatus. J Cell Biol. 1985 May;100(5):1558–1569. doi: 10.1083/jcb.100.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K. E., Palade G. E. Hepatic Golgi fractions resolved into membrane and content subfractions. J Cell Biol. 1982 Mar;92(3):822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero D. R., Lane M. D. Sequential assembly of very low density lipoprotein apolipoproteins, triacylglycerol, and phosphoglycerides by the intact liver cell. J Biol Chem. 1983 Dec 10;258(23):14496–14504. [PubMed] [Google Scholar]
- Kreibich G., Debey P., Sabatini D. D. Selective release of content from microsomal vesicles without membrane disassembly. I. Permeability changes induced by low detergent concentrations. J Cell Biol. 1973 Aug;58(2):436–462. doi: 10.1083/jcb.58.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Pease R. J., Harrison G. B., Scott J. Cotranslocational insertion of apolipoprotein B into the inner leaflet of the endoplasmic reticulum. Nature. 1991 Oct 3;353(6343):448–450. doi: 10.1038/353448a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps G. R., Timko J. L. Simple method for the characterization of 5S RNA. Anal Biochem. 1972 Jan;45(1):319–325. doi: 10.1016/0003-2697(72)90035-8. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., North J. D., Teng B. B., Rifici V. A., Ronhild de Brito A. E., Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res. 1989 Jul;30(7):1065–1077. [PubMed] [Google Scholar]
- RADDING C. M., STEINBERG D. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J Clin Invest. 1960 Oct;39:1560–1569. doi: 10.1172/JCI104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Lucocq J. M., Weinstein J., Paulson J. C. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985 Nov;43(1):287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Siuta-Mangano P., Janero D. R., Lane M. D. Association and assembly of triglyceride and phospholipid with glycosylated and unglycosylated apoproteins of very low density lipoprotein in the intact liver cell. J Biol Chem. 1982 Oct 10;257(19):11463–11467. [PubMed] [Google Scholar]
- Wong L., Pino R. M. Biogenesis of very-low-density lipoproteins in rat liver. Intracellular distribution of apolipoprotein B. Eur J Biochem. 1987 Apr 15;164(2):357–367. doi: 10.1111/j.1432-1033.1987.tb11066.x. [DOI] [PubMed] [Google Scholar]