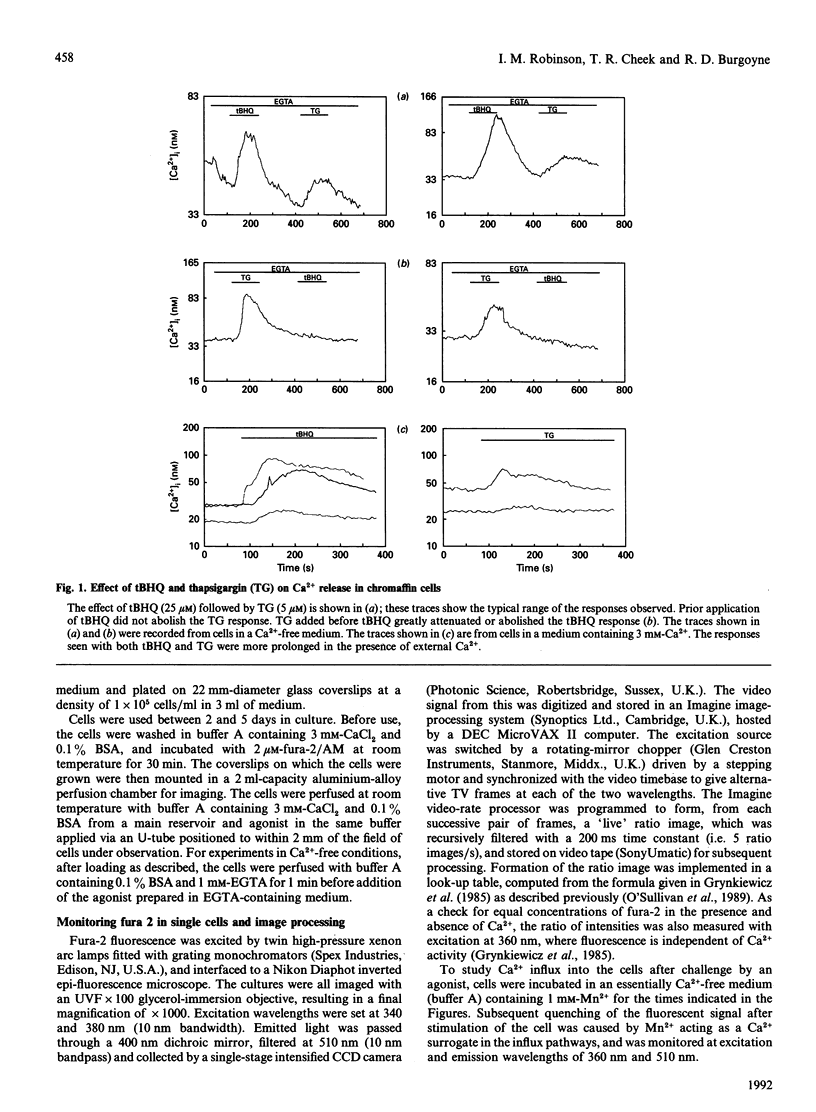
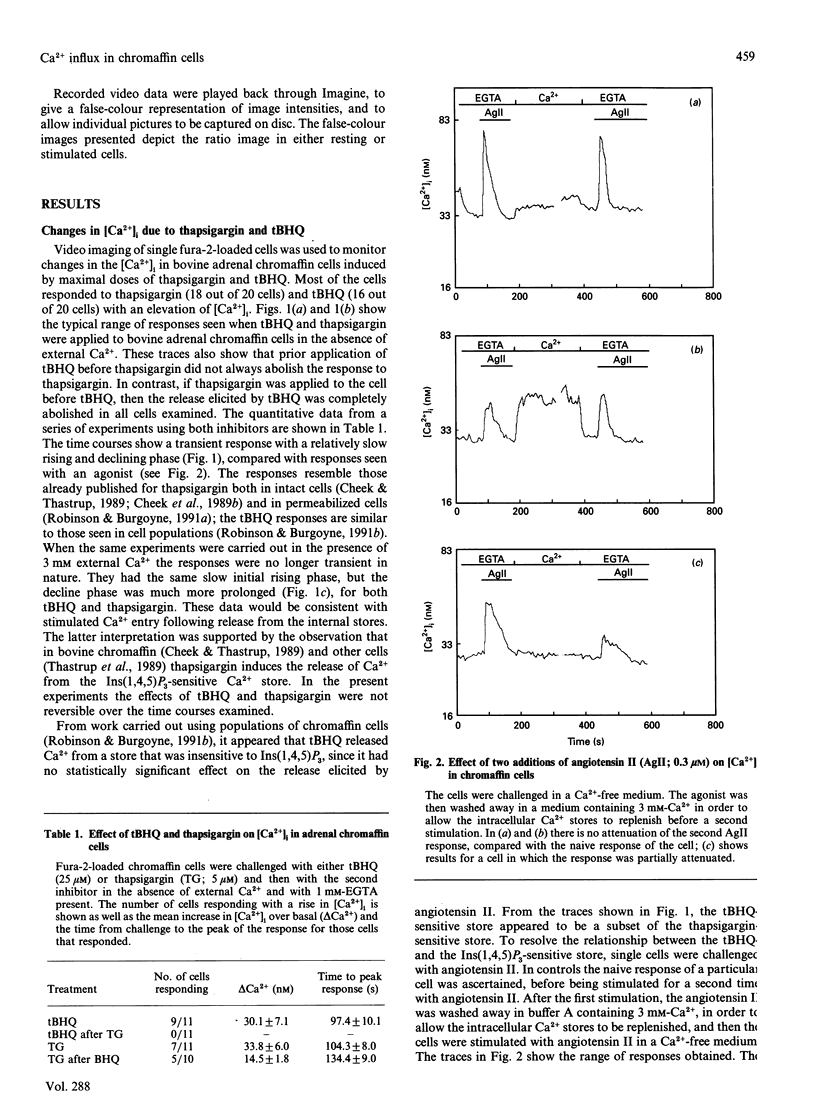
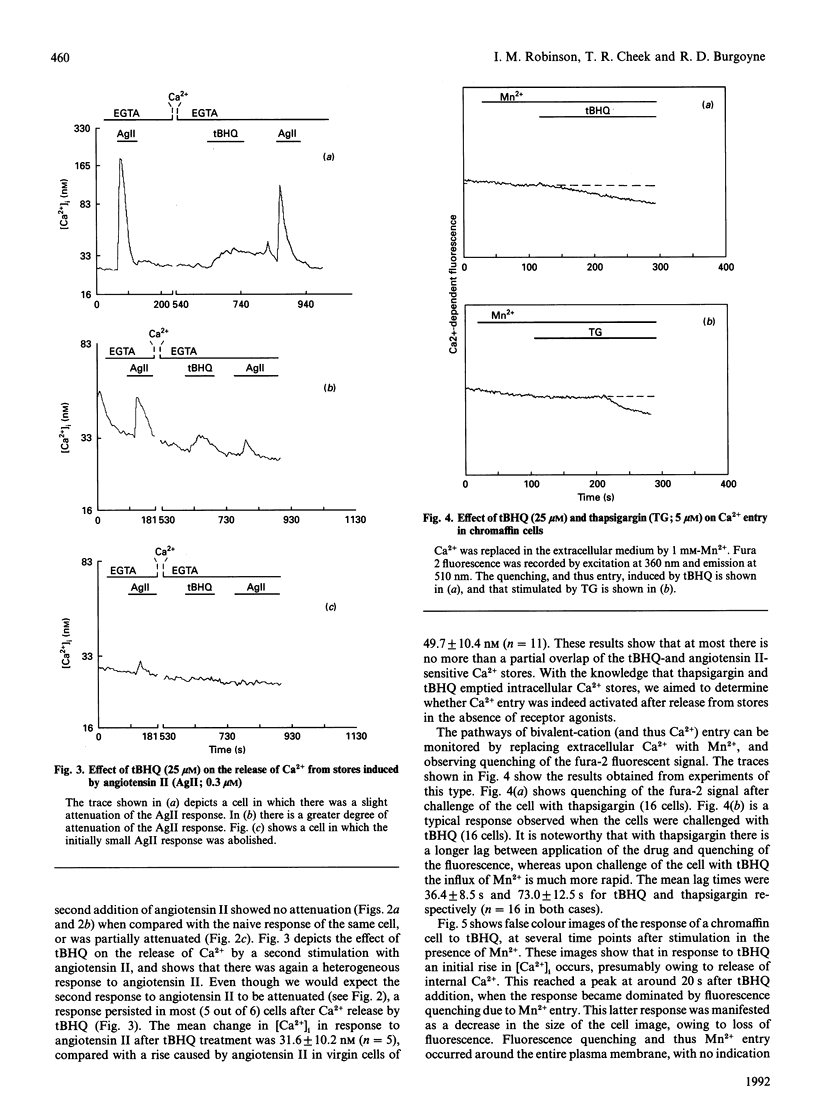
Abstract
We have characterized the effect of the Ca(2+)-ATPase inhibitors 2,5-di-(t-butyl)-1,4-benzohydroquinone (tBHQ) and thapsigargin on the concentration of cytosolic Ca2+ in single bovine adrenal chromaffin cells by video-imaging of fura-2-loaded cells. Addition of either inhibitor released Ca2+ from internal stores in the absence of external Ca2+. tBHQ was unable to stimulate further Ca2+ release after addition of thapsigargin, but thapsigargin could do so after release by tBHQ, indicating that the tBHQ-sensitive stores are a sub-set of those sensitive to thapsigargin. Angiotensin II was able to elicit Ca2+ release after application of tBHQ, indicating that at least part of the tBHQ-sensitive stores were distinct from those discharged by Ins(1,4,5)P3. In the presence of external Ca2+, both Ca(2+)-ATPase inhibitors produced a more prolonged rise in cytosolic Ca2+ consistent with stimulated Ca2+ entry. The ability of the inhibitors to activate a Ca(2+)-entry pathway was confirmed by monitoring quenching of fura-2 after stimulated entry of the Ca2+ surrogate Mn2+. These findings indicate that bovine adrenal chromaffin cells possess a mechanism by which Ca2+ entry can be activated, following emptying of certain internal stores, independently of receptor occupation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Bunn S. J., Marley P. D. Effects of angiotensin II on cultured, bovine adrenal medullary cells. Neuropeptides. 1989 Feb-Mar;13(2):121–132. doi: 10.1016/0143-4179(89)90009-7. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Challis R. A., Jones J. A., Owen P. J., Boarder M. R. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5- tetrakisphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 1991 Mar;56(3):1083–1086. doi: 10.1111/j.1471-4159.1991.tb02033.x. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Jackson T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Simultaneous measurements of cytosolic calcium and secretion in single bovine adrenal chromaffin cells by fluorescent imaging of fura-2 in cocultured cells. J Cell Biol. 1989 Sep;109(3):1219–1227. doi: 10.1083/jcb.109.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells. Distinct nicotinic and muscarinic patterns. FEBS Lett. 1989 Apr 24;247(2):429–434. doi: 10.1016/0014-5793(89)81385-7. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. The caffeine-sensitive Ca2+ store in bovine adrenal chromaffin cells; an examination of its role in triggering secretion and Ca2+ homeostasis. FEBS Lett. 1990 Jun 18;266(1-2):91–95. doi: 10.1016/0014-5793(90)81514-o. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Thastrup O. Internal Ca2+ mobilization and secretion in bovine adrenal chromaffin cells. Cell Calcium. 1989 May-Jun;10(4):213–221. doi: 10.1016/0143-4160(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Dolor R. J., Hurwitz L. M., Mirza Z., Strauss H. C., Whorton A. R. Regulation of extracellular calcium entry in endothelial cells: role of intracellular calcium pool. Am J Physiol. 1992 Jan;262(1 Pt 1):C171–C181. doi: 10.1152/ajpcell.1992.262.1.C171. [DOI] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Cholinergic stimulation of inositol phosphate formation in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic mechanisms. J Neurochem. 1987 Nov;49(5):1634–1643. doi: 10.1111/j.1471-4159.1987.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Roifman C. M., Wong D. Activation of calcium oscillations by thapsigargin in parotid acinar cells. J Biol Chem. 1991 Feb 15;266(5):2778–2782. [PubMed] [Google Scholar]
- Greenberg A., Zinder O. Alpha- and beta-receptor control of catecholamine secretion from isolated adrenal medulla cells. Cell Tissue Res. 1982;226(3):655–665. doi: 10.1007/BF00214792. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. Inositol tetrakisphosphate as a second messenger: confusions, contradictions, and a potential resolution. Bioessays. 1991 Aug;13(8):419–427. doi: 10.1002/bies.950130810. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Inositol(1,3,4,5)tetrakisphosphate-induced activation of sea urchin eggs requires the presence of inositol trisphosphate. Biochem Biophys Res Commun. 1987 Jul 15;146(1):284–290. doi: 10.1016/0006-291x(87)90723-6. [DOI] [PubMed] [Google Scholar]
- Kim K. T., Westhead E. W. Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2+ and secretion from bovine chromaffin cells. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9881–9885. doi: 10.1073/pnas.86.24.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Llopis J., Chow S. B., Kass G. E., Gahm A., Orrenius S. Comparison between the effects of the microsomal Ca(2+)-translocase inhibitors thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone on cellular calcium fluxes. Biochem J. 1991 Jul 15;277(Pt 2):553–556. doi: 10.1042/bj2770553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis J., Kass G. E., Gahm A., Orrenius S. Evidence for two pathways of receptor-mediated Ca2+ entry in hepatocytes. Biochem J. 1992 May 15;284(Pt 1):243–247. doi: 10.1042/bj2840243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Fesce R., Meldolesi J. Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem. 1990 Feb 25;265(6):3005–3008. [PubMed] [Google Scholar]
- Meldolesi J., Clementi E., Fasolato C., Zacchetti D., Pozzan T. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1991 Aug;12(8):289–292. doi: 10.1016/0165-6147(91)90577-f. [DOI] [PubMed] [Google Scholar]
- Mochizuki-Oda N., Mori K., Negishi M., Ito S. Prostaglandin E2 activates Ca2+ channels in bovine adrenal chromaffin cells. J Neurochem. 1991 Feb;56(2):541–547. doi: 10.1111/j.1471-4159.1991.tb08183.x. [DOI] [PubMed] [Google Scholar]
- Moore G. A., McConkey D. J., Kass G. E., O'Brien P. J., Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone--a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS Lett. 1987 Nov 30;224(2):331–336. doi: 10.1016/0014-5793(87)80479-9. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Burgoyne R. D. A comparison of bradykinin, angiotensin II and muscarinic stimulation of cultured bovine adrenal chromaffin cells. Biosci Rep. 1989 Apr;9(2):243–252. doi: 10.1007/BF01116001. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Does inositol tetrakisphosphate play a role in the receptor-mediated control of calcium mobilization? Cell Calcium. 1989 Jul;10(5):375–383. doi: 10.1016/0143-4160(89)90063-8. [DOI] [PubMed] [Google Scholar]
- Preston S. F., Sha'afi R. I., Berlin R. D. Regulation of Ca2+ influx during mitosis: Ca2+ influx and depletion of intracellular Ca2+ stores are coupled in interphase but not mitosis. Cell Regul. 1991 Nov;2(11):915–925. doi: 10.1091/mbc.2.11.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Burgoyne R. D. A distinct 2,5-di-(tert-butyl)-1,4-benzohydroquinone-sensitive calcium store in bovine adrenal chromaffin cells. FEBS Lett. 1991 Sep 9;289(2):151–154. doi: 10.1016/0014-5793(91)81057-f. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Burgoyne R. D. Characterisation of distinct inositol 1,4,5-trisphosphate-sensitive and caffeine-sensitive calcium stores in digitonin-permeabilised adrenal chromaffin cells. J Neurochem. 1991 May;56(5):1587–1593. doi: 10.1111/j.1471-4159.1991.tb02055.x. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. Receptor-mediated calcium entry in fura-2-loaded human platelets stimulated with ADP and thrombin. Dual-wavelengths studies with Mn2+. Biochem J. 1989 Mar 15;258(3):923–926. doi: 10.1042/bj2580923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa N., Nakaki T., Yamamoto S., Kato R. Inositol trisphosphate accumulation by high K+ stimulation in cultured adrenal chromaffin cells. FEBS Lett. 1987 Nov 2;223(2):413–416. doi: 10.1016/0014-5793(87)80330-7. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., McKinney R. A., Murawsky M. M. The role of caffeine-sensitive Ca2+ stores in agonist- and inositol 1,4,5-trisphosphate-induced Ca2+ release from bovine adrenal chromaffin cells. Biochem J. 1991 Sep 15;278(Pt 3):643–650. doi: 10.1042/bj2780643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman K. A., Murawsky M. M., Pruss R. M. Agonist-dependent patterns of cytosolic Ca2+ changes in single bovine adrenal chromaffin cells: relationship to catecholamine release. Cell Regul. 1990 Aug;1(9):683–691. doi: 10.1091/mbc.1.9.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Different patterns of agonist-stimulated increases of 3H-inositol phosphate isomers and cytosolic Ca2+ in bovine adrenal chromaffin cells: comparison of the effects of histamine and angiotensin II. J Neurochem. 1990 Mar;54(3):946–953. doi: 10.1111/j.1471-4159.1990.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Dissociation of Ca2+ entry and Ca2+ mobilization responses to angiotensin II in bovine adrenal chromaffin cells. J Biol Chem. 1989 Nov 5;264(31):18349–18355. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan D. C., Bunn S. J., Livett B. G. Effects of phorbol esters and forskolin on basal and histamine-induced accumulation of inositol phosphates in cultured bovine adrenal chromaffin cells. J Neurochem. 1989 Oct;53(4):1219–1227. doi: 10.1111/j.1471-4159.1989.tb07418.x. [DOI] [PubMed] [Google Scholar]
- Waymire J. C., Bennett W. F., Boehme R., Hankins L., Gilmer-Waymire K., Haycock J. W. Bovine adrenal chromaffin cells: high-yield purification and viability in suspension culture. J Neurosci Methods. 1983 Apr;7(4):329–351. doi: 10.1016/0165-0270(83)90026-2. [DOI] [PubMed] [Google Scholar]