Abstract
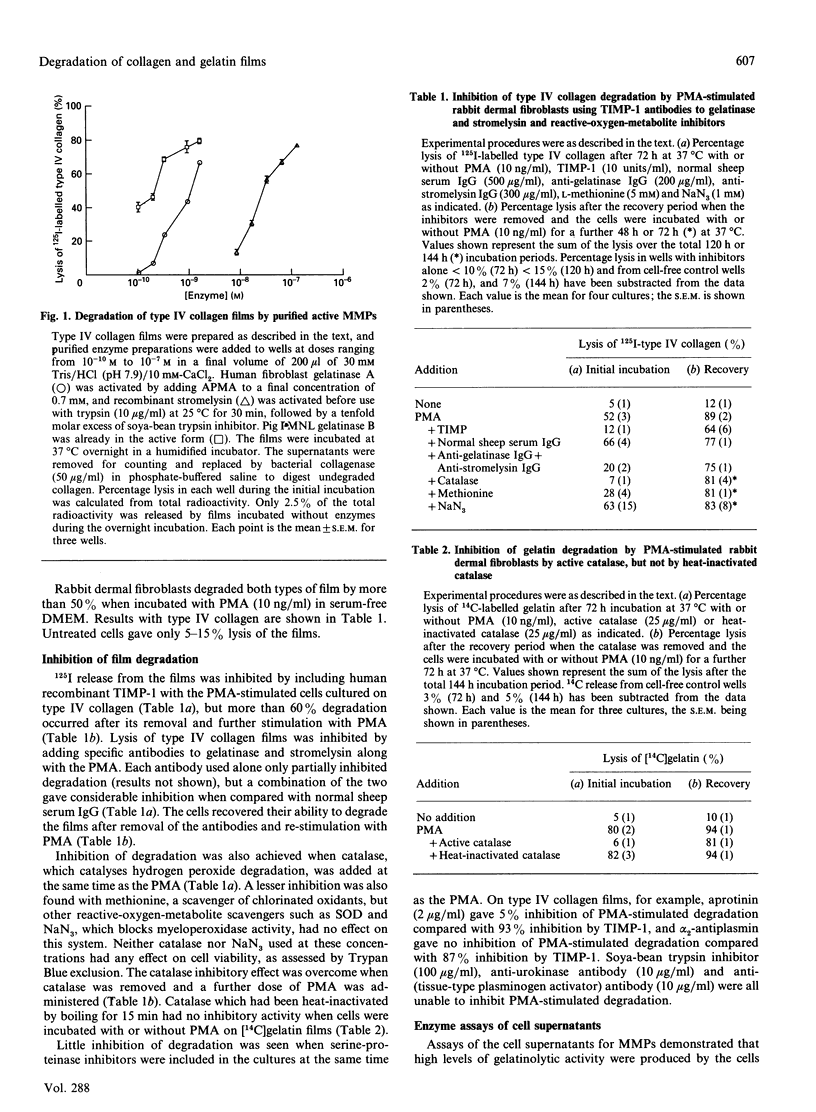
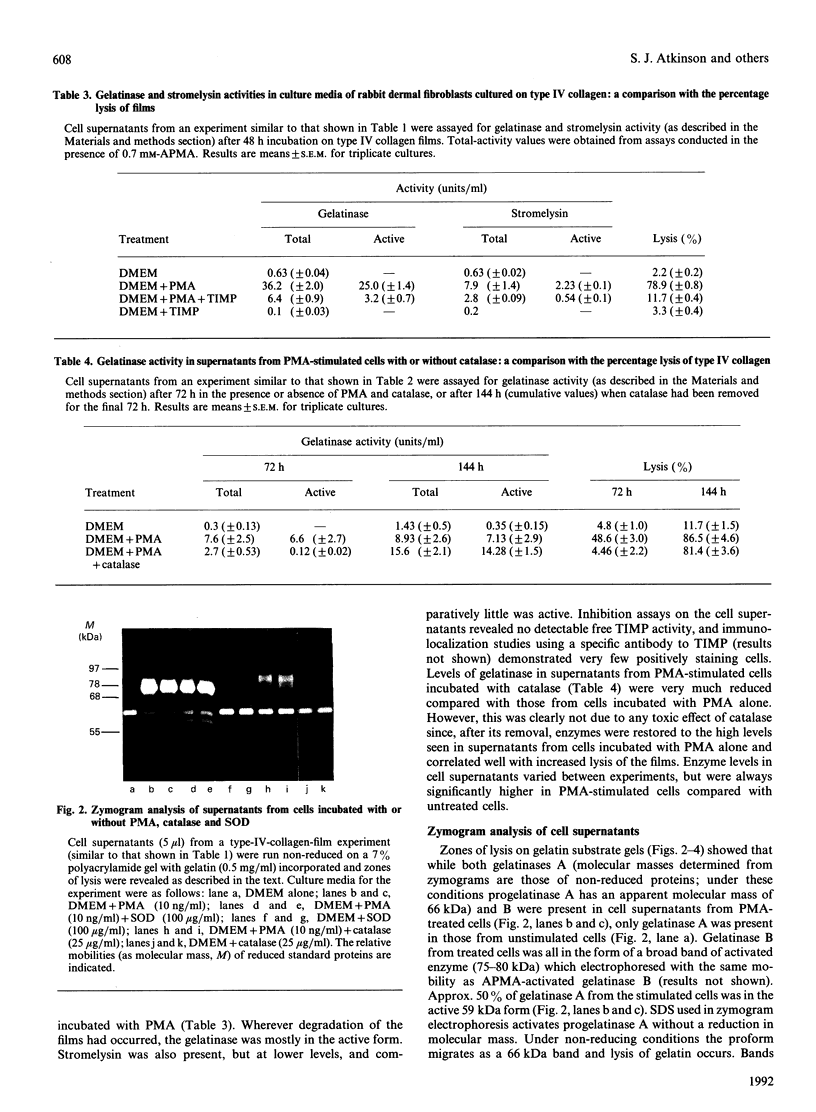
The ability of normal rabbit dermal fibroblasts to degrade films of type IV collagen and gelatin when stimulated by phorbol ester was shown to be dependent on the induction, secretion and activation of 95 kDa gelatinase B and the secretion and activation of 72 kDa gelatinase A and stromelysin. Degradation was inhibited by exogenous human recombinant tissue inhibitor of metalloproteinases-1, specific antibodies to gelatinase and stromelysin and by the reactive-oxygen-metabolite inhibitor catalase. We discuss the various pathways for activation of matrix metalloproteinases in this model system and conclude that, although plasmin may play a key role in the activation of gelatinase B and stromelysin, gelatinase A is activated by a mechanism which has yet to be elucidated. The involvement of oxygen radicals in the direct activation of matrix metalloproteinases in this model is thought to be unlikely.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballin M., Gomez D. E., Sinha C. C., Thorgeirsson U. P. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem Biophys Res Commun. 1988 Aug 15;154(3):832–838. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Johnson C. C., Lowther D. A. Inhibition of proteoglycan synthesis by hydrogen peroxide in cultured bovine articular cartilage. Biochim Biophys Acta. 1985 Feb 15;838(2):221–228. doi: 10.1016/0304-4165(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfil R. D., Reddel R. R., Ura H., Reich R., Fridman R., Harris C. C., Klein-Szanto J. P. Invasive and metastatic potential of a v-Ha-ras-transformed human bronchial epithelial cell line. J Natl Cancer Inst. 1989 Apr 19;81(8):587–594. doi: 10.1093/jnci/81.8.587. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Docherty A. J., Murphy G. The tissue metalloproteinase family and the inhibitor TIMP: a study using cDNAs and recombinant proteins. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):469–479. [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis V., Behrendt N., Danø K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991 Jul 5;266(19):12752–12758. [PubMed] [Google Scholar]
- Garbisa S., Kniska K., Tryggvason K., Foltz C., Liotta L. A. Quantitation of basement membrane collagen degradation by living tumor cells in vitro. Cancer Lett. 1980 Jun;9(4):359–366. doi: 10.1016/0304-3835(80)90030-0. [DOI] [PubMed] [Google Scholar]
- Garbisa S., Pozzatti R., Muschel R. J., Saffiotti U., Ballin M., Goldfarb R. H., Khoury G., Liotta L. A. Secretion of type IV collagenolytic protease and metastatic phenotype: induction by transfection with c-Ha-ras but not c-Ha-ras plus Ad2-E1a. Cancer Res. 1987 Mar 15;47(6):1523–1528. [PubMed] [Google Scholar]
- Gavrilovic J., Hembry R. M., Reynolds J. J., Murphy G. Tissue inhibitor of metalloproteinases (TIMP) regulates extracellular type I collagen degradation by chondrocytes and endothelial cells. J Cell Sci. 1987 Mar;87(Pt 2):357–362. doi: 10.1242/jcs.87.2.357. [DOI] [PubMed] [Google Scholar]
- Gavrilovíc J., Reynolds J. J., Murphy G. Inhibition of type I collagen film degradation by tumour cells using a specific antibody to collagenase and the specific tissue inhibitor of metalloproteinases (TIMP). Cell Biol Int Rep. 1985 Dec;9(12):1097–1107. doi: 10.1016/s0309-1651(85)80007-2. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Hall S. W., Humphries J. E., Gonias S. L. Inhibition of cell surface receptor-bound plasmin by alpha 2-antiplasmin and alpha 2-macroglobulin. J Biol Chem. 1991 Jul 5;266(19):12329–12336. [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hipps D. S., Hembry R. M., Docherty A. J., Reynolds J. J., Murphy G. Purification and characterization of human 72-kDa gelatinase (type IV collagenase). Use of immunolocalisation to demonstrate the non-coordinate regulation of the 72-kDa and 95-kDa gelatinases by human fibroblasts. Biol Chem Hoppe Seyler. 1991 Apr;372(4):287–296. doi: 10.1515/bchm3.1991.372.1.287. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Manchanda N., Schwartz B. S. Single chain urokinase. Augmentation of enzymatic activity upon binding to monocytes. J Biol Chem. 1991 Aug 5;266(22):14580–14584. [PubMed] [Google Scholar]
- Moll U. M., Youngleib G. L., Rosinski K. B., Quigley J. P. Tumor promoter-stimulated Mr 92,000 gelatinase secreted by normal and malignant human cells: isolation and characterization of the enzyme from HT1080 tumor cells. Cancer Res. 1990 Oct 1;50(19):6162–6170. [PubMed] [Google Scholar]
- Monboisse J. C., Poulin G., Braquet P., Randoux A., Ferradini C., Borel J. P. Effect of oxy radicals on several types of collagen. Int J Tissue React. 1984;6(5):385–390. [PubMed] [Google Scholar]
- Murphy G., Atkinson S., Ward R., Gavrilovic J., Reynolds J. J. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann N Y Acad Sci. 1992 Dec 4;667:1–12. doi: 10.1111/j.1749-6632.1992.tb51590.x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cartwright E. C., Sellers A., Reynolds J. J. The detection and characterisation of collagenase inhibitors from rabbit tissues in culture. Biochim Biophys Acta. 1977 Aug 11;483(2):493–498. doi: 10.1016/0005-2744(77)90080-8. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Galloway W. A., Barnes M. J., Bunning R. A., Mercer E., Reynolds J. J., Burgeson R. E. Metalloproteinases from rabbit bone culture medium degrade types IV and V collagens, laminin and fibronectin. Biochem J. 1981 Dec 1;199(3):807–811. doi: 10.1042/bj1990807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Reynolds J. J. An inhibitor of collagenase from human amniotic fluid. Purification, characterization and action on metalloproteinases. Biochem J. 1981 Apr 1;195(1):167–170. doi: 10.1042/bj1950167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., McGarrity A. M., Reynolds J. J., Henderson B. Gelatinase (type IV collagenase) immunolocalization in cells and tissues: use of an antiserum to rabbit bone gelatinase that identifies high and low Mr forms. J Cell Sci. 1989 Mar;92(Pt 3):487–495. doi: 10.1242/jcs.92.3.487. [DOI] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Reynolds J. J. Characterization of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res. 1986 Oct;6(4):351–363. doi: 10.1016/s0174-173x(86)80005-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ward R., Gavrilovic J., Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224–230. [PubMed] [Google Scholar]
- Murphy G., Ward R., Hembry R. M., Reynolds J. J., Kühn K., Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989 Mar 1;258(2):463–472. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Ogata Y., Suzuki K., Enghild J. J., Salvesen G. Substrate specificities and activation mechanisms of matrix metalloproteinases. Biochem Soc Trans. 1991 Aug;19(3):715–718. doi: 10.1042/bst0190715. [DOI] [PubMed] [Google Scholar]
- Okada Y., Morodomi T., Enghild J. J., Suzuki K., Yasui A., Nakanishi I., Salvesen G., Nagase H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990 Dec 27;194(3):721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- Ostrowski L. E., Finch J., Krieg P., Matrisian L., Patskan G., O'Connell J. F., Phillips J., Slaga T. J., Breathnach R., Bowden G. T. Expression pattern of a gene for a secreted metalloproteinase during late stages of tumor progression. Mol Carcinog. 1988;1(1):13–19. doi: 10.1002/mc.2940010106. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Sodek J. Concanavalin A produces a matrix-degradative phenotype in human fibroblasts. Induction and endogenous activation of collagenase, 72-kDa gelatinase, and Pump-1 is accompanied by the suppression of the tissue inhibitor of matrix metalloproteinases. J Biol Chem. 1990 Dec 5;265(34):21141–21151. [PubMed] [Google Scholar]
- Peppin G. J., Weiss S. J. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4322–4326. doi: 10.1073/pnas.83.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopata I., Dancewicz A. M. Presence of a gelatin-specific proteinase and its latent form in human leucocytes. Biochim Biophys Acta. 1974 Dec 29;370(2):510–523. doi: 10.1016/0005-2744(74)90112-0. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Wacher M. P., Margulies I. M., Liotta L. A. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989 Jan 25;264(3):1353–1356. [PubMed] [Google Scholar]
- Thomson B. M., Atkinson S. J., McGarrity A. M., Hembry R. M., Reynolds J. J., Meikle M. C. Type I collagen degradation by mouse calvarial osteoblasts stimulated with 1,25-dihydroxyvitamin D-3: evidence for a plasminogen-plasmin-metalloproteinase activation cascade. Biochim Biophys Acta. 1989 Nov 20;1014(2):125–132. doi: 10.1016/0167-4889(89)90024-4. [DOI] [PubMed] [Google Scholar]
- Tiku M. L., Liesch J. B., Robertson F. M. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J Immunol. 1990 Jul 15;145(2):690–696. [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987 Nov 25;907(3):191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Vissers M. C., Winterbourn C. C. Activation of human neutrophil gelatinase by endogenous serine proteinases. Biochem J. 1988 Jan 15;249(2):327–331. doi: 10.1042/bj2490327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. V., Atkinson S. J., Slocombe P. M., Docherty A. J., Reynolds J. J., Murphy G. Tissue inhibitor of metalloproteinases-2 inhibits the activation of 72 kDa progelatinase by fibroblast membranes. Biochim Biophys Acta. 1991 Aug 30;1079(2):242–246. doi: 10.1016/0167-4838(91)90132-j. [DOI] [PubMed] [Google Scholar]
- Ward R. V., Hembry R. M., Reynolds J. J., Murphy G. The purification of tissue inhibitor of metalloproteinases-2 from its 72 kDa progelatinase complex. Demonstration of the biochemical similarities of tissue inhibitor of metalloproteinases-2 and tissue inhibitor of metalloproteinases-1. Biochem J. 1991 Aug 15;278(Pt 1):179–187. doi: 10.1042/bj2780179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Tanaka R., Ito Y., Shimizu S. Gelatinases of murine metastatic tumor cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):228–234. doi: 10.1016/s0006-291x(89)80202-5. [DOI] [PubMed] [Google Scholar]





