Abstract
The fibroblast cell line L929 contains a constitutively expressed NO synthase (EC 1.14.29.-) activity, which can be increased about 10-fold by tumour-necrosis factor alpha (TNF-alpha). Activities of the constitutive and the inducible enzymes are tetrahydrobiopterin-independent and can be inhibited by L-NG-nitroarginine. Induction of NO synthase by TNF-alpha was prevented by inhibitors of poly(ADP-ribose) polymerase, namely nicotinamide, 3-methoxybenzamide and 3-aminobenzamide. TNF-alpha did not lead to an increase in ADP-ribosyltransferase activity nor to a change in the pattern of ADP-ribosylated proteins. The inhibitors were only active during the first 4-5 h after exposure to TNF-alpha and they were found to suppress synthesis of protein, DNA and RNA. These data suggest that the inhibitors prevent induction of NO synthase by interference with RNA and protein synthesis. It is not yet known which reactions of these biosynthetic processes are affected by the inhibitors.
Full text
PDF
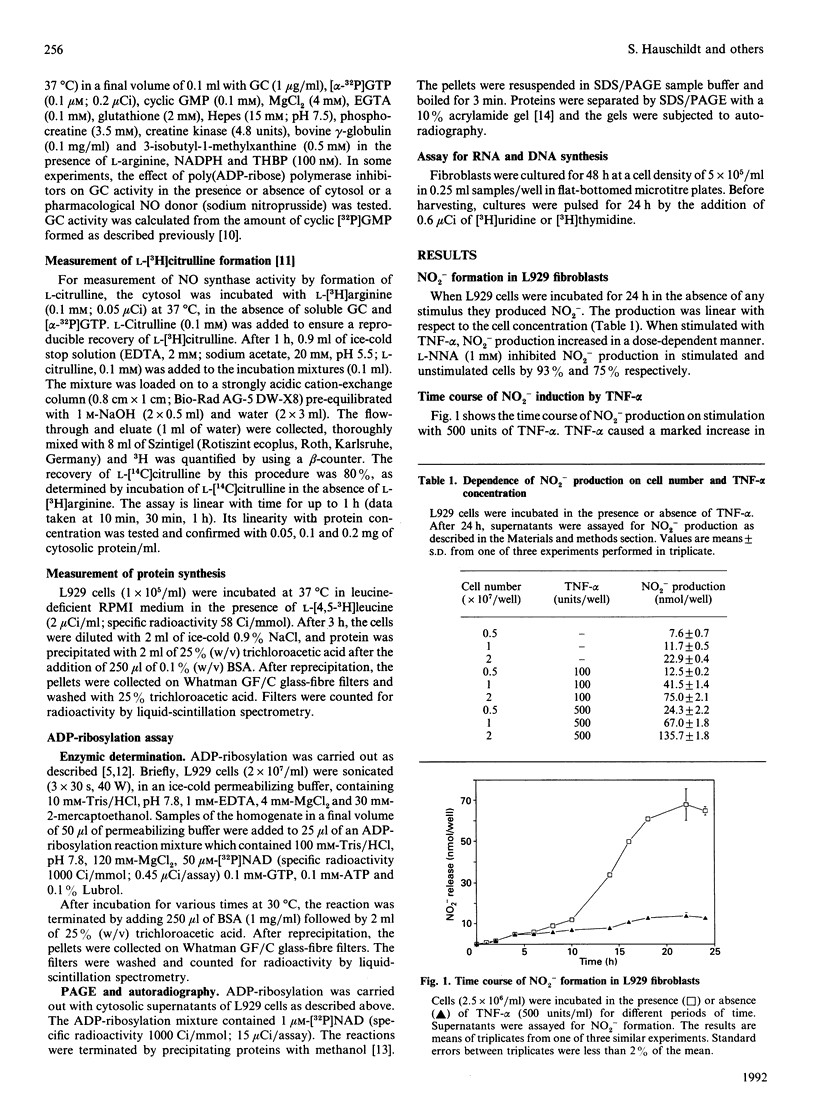
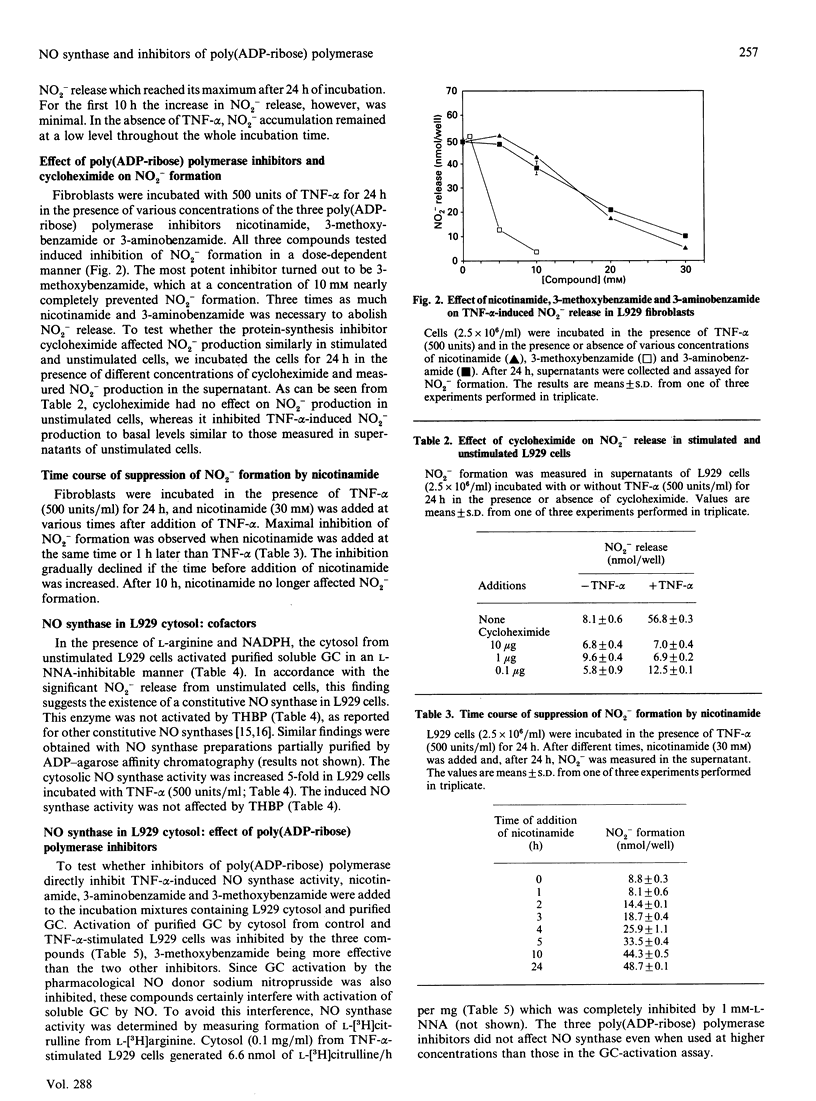
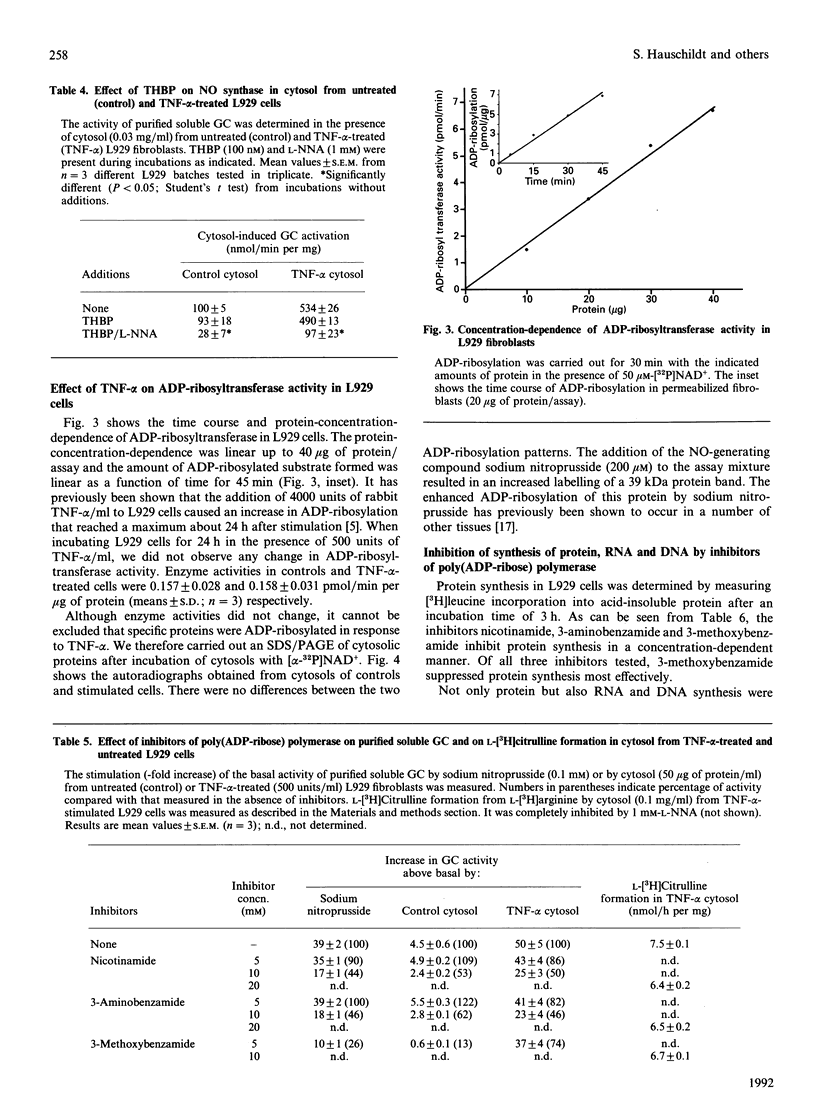


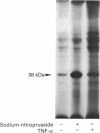
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S., Drysdale B. E., Shin H. S. Tumor necrosis factor-mediated cytotoxicity involves ADP-ribosylation. J Immunol. 1988 Jun 15;140(12):4187–4192. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale B. E., Zacharchuk C. M., Shin H. S. Mechanism of macrophage-mediated cytotoxicity: production of a soluble cytotoxic factor. J Immunol. 1983 Nov;131(5):2362–2367. [PubMed] [Google Scholar]
- Hauschildt S., Bassenge E., Bessler W., Busse R., Mülsch A. L-arginine-dependent nitric oxide formation and nitrite release in bone marrow-derived macrophages stimulated with bacterial lipopeptide and lipopolysaccharide. Immunology. 1990 Jul;70(3):332–337. [PMC free article] [PubMed] [Google Scholar]
- Hauschildt S., Scheipers P., Bessler W. G. Inhibitors of poly (ADP-ribose) polymerase suppress lipopolysaccharide-induced nitrite formation in macrophages. Biochem Biophys Res Commun. 1991 Sep 16;179(2):865–871. doi: 10.1016/0006-291x(91)91898-m. [DOI] [PubMed] [Google Scholar]
- Helson L., Green S., Carswell E., Old L. J. Effect of tumour necrosis factor on cultured human melanoma cells. Nature. 1975 Dec 25;258(5537):731–732. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- Kondo M., Ishida N., Kobayashi M., Mitsui Y. Establishment of a human cell line highly expressing endothelin in serum-free medium. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S52–S54. doi: 10.1097/00005344-199100177-00014. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A., Gera J. F., Andrews J., Berenson J., Ware C. F. Inhibitors of ADP-ribose polymerase decrease the resistance of HER2/neu-expressing cancer cells to the cytotoxic effects of tumor necrosis factor. J Immunol. 1991 Mar 15;146(6):2052–2058. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Mayer B., John M., Böhme E. Purification of a Ca2+/calmodulin-dependent nitric oxide synthase from porcine cerebellum. Cofactor-role of tetrahydrobiopterin. FEBS Lett. 1990 Dec 17;277(1-2):215–219. doi: 10.1016/0014-5793(90)80848-d. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Schmid D. S., Tite J. P., Ruddle N. H. DNA fragmentation: manifestation of target cell destruction mediated by cytotoxic T-cell lines, lymphotoxin-secreting helper T-cell clones, and cell-free lymphotoxin-containing supernatant. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1881–1885. doi: 10.1073/pnas.83.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore C. J., Davies M. I., Goodwin P. M., Halldorsson H., Lewis P. J., Shall S., Zia'ee A. A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979 Nov 1;101(1):135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F. FAD and GSH participate in macrophage synthesis of nitric oxide. Biochem Biophys Res Commun. 1990 Apr 30;168(2):558–565. doi: 10.1016/0006-291x(90)92357-6. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]