Abstract
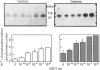
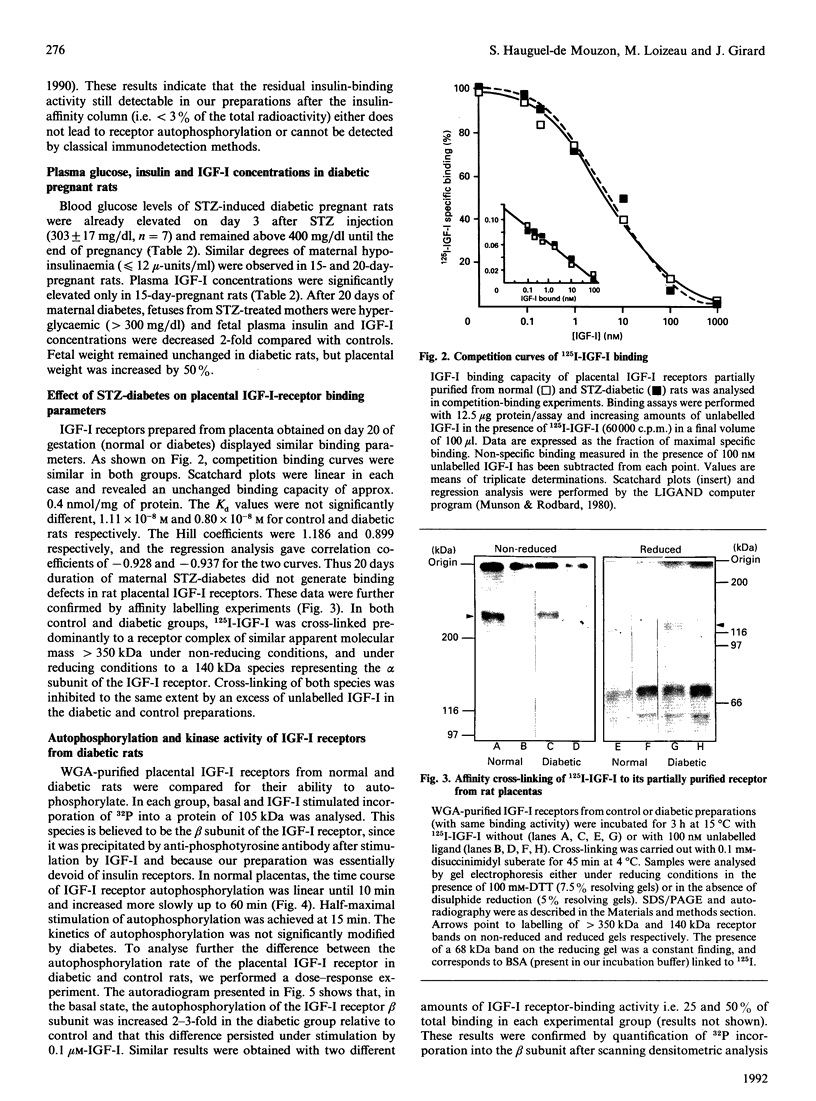
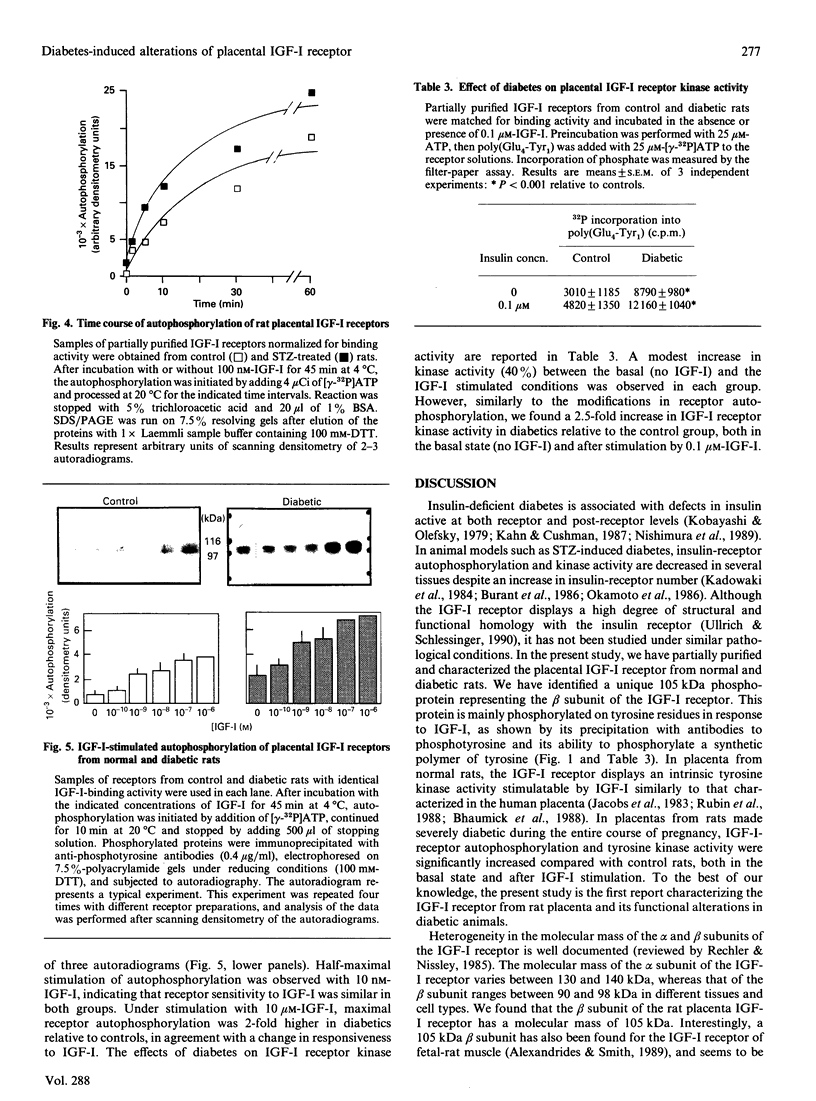
The presence of type I insulin-like growth factor (IGF-I) receptors on placental membranes led to the hypothesis that these receptors might play a critical role in the rapid growth of this organ. Diabetes induces feto-placental overgrowth, but it is not known whether it modifies IGF-I receptor activity in fetal and/or placental tissues. To answer this question, we have partially purified and characterized placental receptors from normal and streptozotocin-induced diabetic rats. In normal rats, binding of 125I-IGF-I to a 140 kDa protein corresponding to the alpha subunit of the receptor was observed in cross-linking experiments performed under reducing conditions. Stimulation by IGF-I induces the autophosphorylation of a 105 kDa phosphoprotein representing the beta subunit of the receptor. In rats made hyperglycaemic and insulinopenic by streptozotocin injection on day 1 of pregnancy, placental IGF-I receptor-binding parameters were not different from controls on day 20 of pregnancy. In contrast, the autophosphorylation and kinase activity of IGF-I receptors of diabetic rats were increased 2-3-fold in the basal state and after IGF-I stimulation. The present study indicates that the rat placental IGF-I receptor possesses structural characteristics similar to that reported for fetal-rat muscle, and suggests that the high-molecular-mass beta subunit could represent a type of receptor specifically expressed during prenatal development. In addition, it clearly demonstrates that diabetes induces functional alterations in IGF-I receptor kinase activity that may play a major role in the placental overgrowth in diabetic pregnancy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrides T. K., Smith R. J. A novel fetal insulin-like growth factor (IGF) I receptor. Mechanism for increased IGF I- and insulin-stimulated tyrosine kinase activity in fetal muscle. J Biol Chem. 1989 Aug 5;264(22):12922–12930. [PubMed] [Google Scholar]
- Bhaumick B., Danilkewich A. D., Bala R. M. Altered placental insulin and insulin-like growth factor-I receptors in diabetes. Life Sci. 1988;42(17):1603–1614. doi: 10.1016/0024-3205(88)90439-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnol A. F., Loizeau M., Girard J. Insulin receptor activity and insulin sensitivity in mammary gland of lactating rats. Am J Physiol. 1990 Dec;259(6 Pt 1):E828–E834. doi: 10.1152/ajpendo.1990.259.6.E828. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Applewhite G. T., Underwood L. E. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol. 1980 Mar 15;75(2):315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Mariz I. K., Trivedi B. A preferential binding site for insulin-like growth factor II in human and rat placental membranes. J Clin Endocrinol Metab. 1981 Aug;53(2):282–288. doi: 10.1210/jcem-53-2-282. [DOI] [PubMed] [Google Scholar]
- Deal C. L., Guyda H. J., Lai W. H., Posner B. I. Ontogeny of growth factor receptors in the human placenta. Pediatr Res. 1982 Oct;16(10):820–826. doi: 10.1203/00006450-198210000-00004. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Klein H. H., Cordera R., Olefsky J. M. Insulin receptor kinase activity in rat liver. Regulation by fasting and high carbohydrate feeding. J Biol Chem. 1985 Oct 15;260(23):12444–12453. [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Ballotti R., Kowalski A., Westermark B., Van Obberghen E. Expression of two types of receptor for insulin-like growth factors in human malignant glioma. Cancer Res. 1988 Mar 1;48(5):1233–1237. [PubMed] [Google Scholar]
- Garofalo R. S., Rosen O. M. Insulin and insulinlike growth factor 1 (IGF-1) receptors during central nervous system development: expression of two immunologically distinct IGF-1 receptor beta subunits. Mol Cell Biol. 1989 Jul;9(7):2806–2817. doi: 10.1128/mcb.9.7.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. R., Cuendet G. S., Marliss E. B., Kervran A., Rieutort M., Assan R. Fuels, hormones, and liver metabolism at term and during the early postnatal period in the rat. J Clin Invest. 1973 Dec;52(12):3190–3200. doi: 10.1172/JCI107519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D. B., Masaracchia R. A., Feramisco J. R., Kemp B. E. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal Biochem. 1978 Jul 1;87(2):566–575. doi: 10.1016/0003-2697(78)90707-8. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Itin A. Purification of the insulin receptor from human placenta by chromatography on immobilized wheat germ lectin and receptor antibody. J Biol Chem. 1980 Dec 25;255(24):12066–12072. [PubMed] [Google Scholar]
- Heydrick S. J., Ruderman N. B., Kurowski T. G., Adams H. B., Chen K. S. Enhanced stimulation of diacylglycerol and lipid synthesis by insulin in denervated muscle. Altered protein kinase C activity and possible link to insulin resistance. Diabetes. 1991 Dec;40(12):1707–1711. doi: 10.2337/diab.40.12.1707. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Phosphorylation of receptors for insulin and insulin-like growth factor I. Effects of hormones and phorbol esters. J Biol Chem. 1986 Jan 15;261(2):934–939. [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Jonas H. A., Harrison L. C. The human placenta contains two distinct binding and immunoreactive species of insulin-like growth factor-I receptors. J Biol Chem. 1985 Feb 25;260(4):2288–2294. [PubMed] [Google Scholar]
- Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984 Nov 25;259(22):14208–14216. [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Mechanism for markedly hyperresponsive insulin-stimulated glucose transport activity in adipose cells from insulin-treated streptozotocin diabetic rats. Evidence for increased glucose transporter intrinsic activity. J Biol Chem. 1987 Apr 15;262(11):5118–5124. [PubMed] [Google Scholar]
- Kasuga M., Van Obberghen E., Nissley S. P., Rechler M. M. Demonstration of two subtypes of insulin-like growth factor receptors by affinity cross-linking. J Biol Chem. 1981 Jun 10;256(11):5305–5308. [PubMed] [Google Scholar]
- Kellerer M., Obermaier-Kusser B., Ermel B., Wallner U., Häring H. U., Petrides P. E. An altered IGF-I receptor is present in human leukemic cells. J Biol Chem. 1990 Jun 5;265(16):9340–9345. [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Effects of streptozotocin-induced diabetes on insulin binding, glucose transport, and intracellular glucose metabolism in isolated rat adipocytes. Diabetes. 1979 Feb;28(2):87–95. doi: 10.2337/diab.28.2.87. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. S., MacGregor L. C., Fluharty S. J., King G. L. Differential regulation of protein kinase C and (Na,K)-adenosine triphosphatase activities by elevated glucose levels in retinal capillary endothelial cells. J Clin Invest. 1989 Jan;83(1):90–94. doi: 10.1172/JCI113889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee T. S., Saltsman K. A., Ohashi H., King G. L. Activation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complications. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5141–5145. doi: 10.1073/pnas.86.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Leturque A., Burnol A. F., Ferré P., Girard J. Pregnancy-induced insulin resistance in the rat: assessment by glucose clamp technique. Am J Physiol. 1984 Jan;246(1 Pt 1):E25–E31. doi: 10.1152/ajpendo.1984.246.1.E25. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Backer J. M., Kahn C. R. Hepatic phosphotyrosine phosphatase activity and its alterations in diabetic rats. J Clin Invest. 1989 Sep;84(3):976–983. doi: 10.1172/JCI114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Jarnagin K., Roth R. A. Purification and characterization of the receptor for insulin-like growth factor I. Biochemistry. 1986 Sep 23;25(19):5560–5564. doi: 10.1021/bi00367a032. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Kuzuya H., Okamoto M., Yamada K., Kosaki A., Kakehi T., Inoue G., Kono S., Imura H. Postreceptor defect in insulin action in streptozotocin-induced diabetic rats. Am J Physiol. 1989 May;256(5 Pt 1):E624–E630. doi: 10.1152/ajpendo.1989.256.5.E624. [DOI] [PubMed] [Google Scholar]
- Okamoto M., White M. F., Maron R., Kahn C. R. Autophosphorylation and kinase activity of insulin receptor in diabetic rats. Am J Physiol. 1986 Nov;251(5 Pt 1):E542–E550. doi: 10.1152/ajpendo.1986.251.5.E542. [DOI] [PubMed] [Google Scholar]
- Okumura K., Akiyama N., Hashimoto H., Ogawa K., Satake T. Alteration of 1,2-diacylglycerol content in myocardium from diabetic rats. Diabetes. 1988 Sep;37(9):1168–1172. doi: 10.2337/diab.37.9.1168. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Rubin J. B., Shia M. A., Pilch P. F. Stimulation of tyrosine-specific phosphorylation in vitro by insulin-like growth factor I. 1983 Sep 29-Oct 5Nature. 305(5933):438–440. doi: 10.1038/305438a0. [DOI] [PubMed] [Google Scholar]
- Sara V. R., Hall K., Misaki M., Fryklund L., Christensen N., Wetterberg L. Ontogenesis of somatomedin and insulin receptors in the human fetus. J Clin Invest. 1983 May;71(5):1084–1094. doi: 10.1172/JCI110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen S. E., Thompson K., Petersen D. J. Separation of the high affinity insulin-like growth factor I receptor from low affinity binding sites by affinity chromatography. J Biol Chem. 1987 Dec 5;262(34):16461–16469. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- van de Werve G., Zaninetti D., Lang U., Vallotton M. B., Jeanrenaud B. Identification of a major defect in insulin-resistant tissues of genetically obese (fa/fa) rats. Impaired protein kinase C. Diabetes. 1987 Mar;36(3):310–314. doi: 10.2337/diab.36.3.310. [DOI] [PubMed] [Google Scholar]