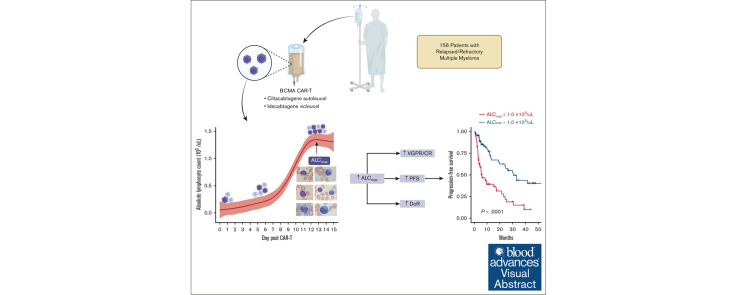
Key Points
-
•
ALCmax within 15 days of BCMA CAR-T infusion is associated with deeper response and occurrence of cytokine release syndrome.
-
•
ALCmax <0.5 ×103/μL is predictive of nonresponders, whereas that of >1.0 × 103/μL is an independent biomarker for sustained response.
Visual Abstract
Abstract
B-cell maturation antigen (BCMA)–targeting chimeric antigen receptor T cells (CAR-Ts) used in multiple myeloma (MM) are rapidly becoming a mainstay in the treatment of relapsed/refractory (R/R) disease, and CAR-T expansion after infusion has been shown to inform depth and duration of response (DoR), but measuring this process remains investigational. This multicenter study describes the kinetics and prognostic impact of absolute lymphocyte count (ALC) in the first 15 days after CAR-T infusion in 156 patients with relapsed MM treated with the BCMA-targeting agents ciltacabtagene autoleucel and idecabtagene vicleucel. Patients with higher maximum ALC (ALCmax) had better depth of response, progression-free survival (PFS), and DoR. Patients with ALCmax >1.0 × 103/μL had a superior PFS (30.5 months vs 6 months; P < .001) compared with those with ≤1.0 × 103/μL, whereas patients with ALCmax ≤0.5 × 103/μL represent a high-risk group with early disease progression and short PFS (hazard ratio, 3.4; 95% confidence interval, 2-5.8; P < .001). In multivariate analysis, ALCmax >1.0 × 103/μL and nonparaskeletal extramedullary disease were the only independent predictors of PFS and DoR after accounting for international staging systemic staging, age, CAR-T product, high-risk cytogenetics, and the number of previous lines. Moreover, our flow cytometry data suggest that ALC is a surrogate for BCMA CAR-T expansion and can be used as an accessible prognostic marker. We report, to our knowledge, for the first time the association of ALC after BCMA CAR-T infusion with clinical outcomes and its utility in predicting response in patients with R/R MM.
Introduction
Chimeric antigen receptor T-cell (CAR-T) therapy has emerged as an effective therapeutic approach in multiple hematological malignancies, such as relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) and non-Hodgkin lymphomas (NHLs) such as diffuse large B-cell lymphoma and low-grade NHL. In multiple myeloma (MM), 2 B-cell maturation antigen (BCMA)–targeting CAR-T products, ciltacabtagene autoleucel (cilta-cel) and idecabtagene vicleucel (ide-cel), are currently approved for use by the Food and Drug Administration and European Medicines Agency in the R/R MM setting, with overall response rates >70% in heavily pretreated patients and with responders often displaying a prolonged progression-free survival (PFS).1, 2, 3, 4 However, the duration of response (DoR) can be highly variable among patients with R/R MM after CAR-T therapy, and there is a need to better understand factors that portend better outcomes. The presence of extramedullary disease (EMD) and high-risk cytogenetic alterations have been associated with poor outcomes, whereas negative minimal residual disease has been associated with improved outcomes, but beyond these, additional prognostic variables are urgently needed to better understand what distinguishes patients enjoying prolonged post–CAR-T remissions from those with short-lived ones.5, 6, 7
After infusion, in vivo CAR-T expansion is seen across all CAR-T constructs and diseases, and the degree of CAR-T expansion and persistence are directly related to efficacy as well as immune-mediated toxicities such as cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS). Furthermore, the composition of T lymphocytes in the CAR-T product or the expanding CAR-T population may affect outcomes, because higher CD4 T-cell subsets have been linked to longer responses with CD19-directed CAR-Ts in B-ALL.8, 9, 10 However, techniques to measure CAR-T expansion and persistence are limited to research settings and lacks prognostic value when determining response. Considering the commercial use of CAR-Ts in R/R MM, widely available surrogates for CAR-T expansion that could predict depth and durability of response are essential.
Although CAR-T expansion is a well-known phenomenon, overt lymphocytosis in the peripheral blood after CAR-T infusion has not been widely described. An increase in absolute lymphocyte count (ALC) within 2 weeks after CAR-T infusion has been described in R/R B-ALL treated with CD19-targeting CAR-Ts, with a peak ALC at day +10 and patients with sustained responses having higher peak ALCs.11 However, this has not been described in other disease entities treated with CAR-T therapy. Here, we describe the kinetics of ALC after BCMA CAR-T infusion and the relationship of these kinetics to both immune-mediated toxicities and clinical outcomes in patients with MM receiving BCMA CAR-T. Additionally, we compare the ALC kinetics of these BCMA-directed products with that of CD19 CAR-Ts in NHL to further illustrate product-specific differences.
Methods
BCMA CAR-T MM cohort
All patients with R/R MM who received cilta-cel or ide-cel at our 3 institutions (Weill Cornell Medicine, Columbia University Irving Medical Center, and Mount Sinai Hospital) were included. Baseline demographics and clinical characteristics, including ALC at preconditioning and throughout days 0 to +15 after CAR-T infusion, were collected for each patient. Immune-related events (CRS and ICANS) during follow-up were graded based on the American Society for Transplant and Cellular Therapy classification.12 Best response was assessed only for those with >3 months of follow-up, because serological response may lag behind complete bone marrow response, which may lead to early discordant bone marrow–serological response assessment after BCMA CAR-T.13 All patients with at least 30 days of follow-up after CAR-T infusion were assessed for disease course and progression. Time to first response, time to best response, DoR, and PFS were measured, with International Myeloma Working Group response criteria used to assess the depth of response and progressive disease (PD).14 Infectious events were defined as nonneutropenic infections that were adjudicated on retrospective review, with or without microbiological isolate on days 0 through 30. Immune effector cell–associated hematotoxicity delayed cytopenia were defined as 2 consecutive time points below the defined threshold for absolute neutrophil count (ANC), platelets, and hemoglobin during days 30 through 60. Cytopenia grading was based on immune effector cell–associated hematotoxicity European Hematology Association/European Bone Marrow Transplant consensus in the case of neutropenia15 and Common Terminology Criteria for Adverse Events version 5 for thrombocytopenia and anemia.
CD19 CAR-T NHL cohort
ALCs from day –5 to day +15 of all patients who received axicabtagene ciloleucel, lisocabtagene maraleucel, tisagenlecleucel, or brexucabtagene autoleucel CD19 CAR-T for NHL were included. NHL subtype, CD19 CAR-T product used, and best response according to Lugano lymphoma response classification16 were retrospectively collected when available.
ALC kinetics indicators
To characterize the ALC kinetics, the maximum ALC (ALCmax; defined as the highest ALC from days 0 through 15), baseline ALC (defined as the lowest ALC during days 0 through +2), absolute change in ALC (defined as the difference between ALCmax and baseline [day 0 ALC]), and preconditioning ALC (defined as the closest ALC to day –5 preceding the start of lymphodepleting conditioning) were assessed for each patient. ALCmax was analyzed as a continuous variable and using the cutoff points based on ALC reference range (>1.0 × 103 to >3.74 × 103/μL).
Peripheral blood flow cytometry
Peripheral blood cells were processed using the label-lyse-wash protocol or a density gradient purification (Ficoll 400, Sigma-Aldrich) and washed and counted (Nexcelom Bioscience). Approximately 0.5 × 106 to 1 × 106 cells were first incubated with Fc blocker (BD Pharmingen) and then the rhBCMA-Fc (R&D systems) labeled fusion protein, and a monoclonal antibody cocktail (supplemental Table 1) was added. Cells were incubated at RT (room temperature) for 15 minutes in the dark, then washed and analyzed on a BD FACSCanto (BD Biosciences, San Jose, CA), equipped with the FACSDiva software (BD Biosciences).
Statistical analysis
Baseline characteristics were described using descriptive statistics and compared between cilta-cel and ide-cel. All patients with available ALC data from days –5 to +15 were included in the initial analysis of ALC dynamics. Relative frequencies were used to describe categorical variables, whereas median and interquartile range in parentheses were used to describe continuous variables unless indicated otherwise. Nonparametric hypothesis tests were used to compare differences in characteristics between groups. The relationship of baseline variables with ALC dynamics was explored using regression as indicated.
Time-to-event analysis was used to assess PFS, which was defined as the time from CAR-T infusion to progression of disease based on International Myeloma Working Group criteria or death. Follow-up time was calculated using the reverse Kaplan-Meier method. PFS distribution was estimated using the Kaplan-Meier method. Differences in PFS were compared using log-rank test and Cox regression. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox regression. Univariable Cox regression was used to identify variables associated with PFS, and all variables associated with PFS were included in a multivariable PFS Cox model after a step-by-step forward inclusion of independent variables associated with P values <.1 in the univariable model. All tests were 2-sided, with a P value <.05 considered as statistically significant. All analysis was performed using R software (R Foundation for Statistical Computing, Vienna, Austria).
The data was obtained after approval of the study protocols by the institutional review boards and of the participating institutions (IRB#22-01024290, 0010004608, and 24-00441). All relevant ethical regulations were followed, and all the research was conducted in accordance with the Declaration of Helsinki.
Results
Patient characteristics
A total of 156 patients received BCMA CAR-T between 2017 and 2023, with a higher proportion of ide-cel patients receiving infusion during 2017 to 2018 (28% vs 3.2%) and a higher proportion of cilta-cel patients (63% vs 26%) in the 2022 to 2023 period. In this cohort, 48% of patients received BCMA CAR-T in an off-trial setting, whereas 52% were part of clinical trials and received the recommended phase 2 dose. Baseline characteristics, including age at infusion, international staging systemic, presence of EMD, and presence of high-risk cytogenetics (deletion 17p (del17p), t(14;16), t(4;14), and gain 1q) were comparable across patients receiving the 2 BCMA CAR-T products. Other baseline characteristics can be found in Table 1. The incidence of CRS and ICANS were similar to those previously reported for cilta-cel2 and ide-cel,1 and there was no difference in the incidence of CRS/ICANS between the products (CRS, 88% vs 86%[P = .75] and ICANS 13% vs 11% [P = .65] for cilta-cel and ide-cel, respectively). Most cases of CRS were grade 1 (73% for cilta-cel and 76% for ide-cel). The median follow-up time of the cohort was 17.5 months (range, 1-58) for all patients, 9.4 months (range, 1-54.7) for cilta-cel, and 33.2 months (range, 2.3-58) for ide-cel. Only 10 patients (1 ide-cel and 9 cilta-cel) had a follow-up of <3 months. The overall response rate was 89%, with complete response (CR) being achieved by 65% (n = 104) of patients, and 46% (n = 72) achieved minimal residual disease negativity in the whole cohort. See Table 1 for details.
Table 1.
Baseline characteristics of the BCMA CAR-T cohort
| Variable | All patients N = 156 |
CAR-T product |
P value∗ | |
|---|---|---|---|---|
| Ide-cel n = 65 |
Cilta-cel n = 91 |
|||
| Age at diagnosis median (IQR) | 55 (48-62) | 54 (48-58) | 55 (48-66) | .51 |
| Age at CAR-T infusion median (IQR) | 62 (55-69) | 61 (53-68) | 63 (56-71) | .19 |
| Secretory type, n (%) | ||||
| Heavy and light chain | 147 (94) | 62 (95) | 85 (93) | .74 |
| Light chain only | 9 (5.8) | 3 (4.6) | 6 (6.6) | |
| Presence of high-risk CG,†n (%) | ||||
| No high-risk CG | 54 (35) | 22 (34) | 32 (35) | .86 |
| High-risk CG | 102 (65) | 43 (66) | 59 (65) | |
| Heavy chain isotype, n (%) | ||||
| IgA | 30 (19) | 13 (20) | 17 (19) | .96 |
| IgG | 89 (58) | 37 (58) | 52 (58) | |
| Light chain only | 35 (23) | 14 (22) | 21 (23) | |
| Year CAR-T infusion, n (%) | ||||
| 2017-2018 | 21 (13) | 18 (28) | 3 (3.3) | <.001 |
| 2019-2021 | 60 (38) | 30 (46) | 30 (33) | |
| 2022-2023 | 75 (48) | 17 (26) | 58 (64) | |
| EMD | ||||
| EMD present | 65 (42) | 23 (35) | 42 (46) | .18 |
| EMD not present | 91 (58) | 42 (65) | 49 (54) | |
| Type of EMD, n (%) | ||||
| Nonparaskeletal | 20 (31) | 4 (17) | 16 (38) | .16 |
| Paraskeletal | 29 (45) | 11 (48) | 18 (43) | |
| Paraskeletal and nonparaskeletal | 16 (25) | 8 (35) | 8 (19) | |
| ISS stage, n (%) | ||||
| Stage I | 55 (44) | 22 (45) | 33 (43) | .73 |
| Stage II | 40 (32) | 17 (35) | 23 (30) | |
| Stage III | 30 (24) | 10 (20) | 20 (26) | |
| Best response after CAR-T, n (%) | ||||
| CR | 99 (64.2) | 36 (55) | 63 (72) | .2 |
| VGPR | 21 (13.6) | 11 (17) | 10 (11) | |
| PR | 17 (11) | 8 (12) | 9 (9.1) | |
| SD/PD | 17 (11) | 10 (15) | 7 (8) | |
| Autologous stem cell transplant, n (%) | 133 (84) | 55 (85) | 78 (84) | .85 |
| Presence of CRS, n (%) | 136 (87.1) | 56 (86) | 80 (88) | .75 |
| Presence of ICANS, n (%) | 19 (12.1) | 7 (11) | 12 (13) | .65 |
CG, cytogenetics; IgA, immunoglobin A; IQR, interquartile range; ISS, international staging systemic.
Pearson χ2 test, Wilcoxon rank sum test, and Fisher exact test as appropriate.
High-risk cytogenetics were defined as deletion 17p/t(4;14)/t(14;16)/gain 1q.
ALC kinetics after BCMA CAR-T
Individual ALC values before lymphodepleting conditioning (day –5) and from days 0 to +15 for the entire cohort and for each BCMA CAR-T product are shown in supplemental Figure 1A-B. Overall, an increase in ALC after days +6 to +7 was seen with both products, although there were notable differences in ALCmax and time to ALCmax between ide-cel and cilta-cel (supplemental Figure 1C). The median preconditioning ALC was 0.83 × 103/μL (0.5× 103 to 1.2 × 103/μL) for ide-cel and 1.0 × 103/μL (0.5 × 103 to 1.3× 103/μL) for cilta-cel (P = .6), whereas the median ALCmax for all patients was 1.28 × 103/μL (0.7 × 103 to 2.7 × 103/μL); however, patients treated with cilta-cel had a higher median ALCmax than those treated with ide-cel (2 × 103/μL [0.99 × 103 to 4.4 × 103/μL] vs 0.8 × 103/uL [0.50 × 103 to 1.1 × 103/μL]; P < .001). The absolute change in ALC was 1.28 × 103/μL (0.6 × 103 to 2.9 × 103/μL) for the entire cohort, with cilta-cel patients exhibiting higher absolute change than ide-cel patients (2.1 × 103/μL [0.9 × 103 to 4.4 × 103/μL] vs 0.77 × 103/μL [0.4 × 103 to 1.1 × 103/μL]; P < .001). Using 3.75 × 103/μL as the upper limit of normal for ALC, 32% of cilta-cel–treated patients developed lymphocytosis compared with none of those treated with ide-cel. Similarly, 74% and 29% of patients treated with cilta-cel and ide-cel, respectively, had an ALCmax >1.0 × 103/μL (P < .001; supplemental Table 2).
Patients who received ide-cel had a shorter time to ALCmax than patients treated with cilta-cel (median time to ALCmax day +11 [9-13] vs day +12 [11-13]; P = .002). Differences in the timing of the ALC rise were seen between CAR-T products when median ALC at each day was compared between products with higher median ALC at days +6 and +7 for ide-cel, followed by higher median ALC for those treated with cilta-cel on days +11 through +15. Conversely, there were no differences seen in the median preconditioning ALC or in the baseline ALC between the 2 BCMA CAR-T cohorts. Additionally, preconditioning ALC and ALCmax had a very weak (r = 0.17) correlation that was not statistically significant (P = .053; supplemental Figure 2).
Presence of ICANS and CRS and absolute lymphocyte change
Association of several baseline variables with ALCmax was explored using univariable linear regression, both for each CAR-T cohort and as a pooled cohort. The presence of CRS was associated with higher ALCmax for all patients with an odds ratio (OR) of 5.16 (95% CI, 1.4-18.7; P = .018) and for those receiving cilta-cel (OR, 12.6; 95% CI, 1.7-93; P = .013). There was an association trend between ALCmax and presence of CRS in the ide-cel group but was not statistically significant (OR, 1.38; 95% CI, 0.77-1.7; P = .1) Similarly, the presence of ICANS was associated with higher ALCmax in the pooled cohorts (OR, 6.6; 95% CI, 1.8-24.5; P = .005) and in both cilta-cel (OR, 13; 95% CI, 1.9-90.1; P = .009) and ide-cel (OR, 1.57; 95% CI, 1.03-2.03; P = .03) groups. Interestingly, patients who developed late neurotoxicity had higher ALCmax than those who did not (4.3 × 103/μL vs 1.1 × 103/μL; P = .02).
Moreover, higher grade of CRS was also associated with higher ALCmax in the pooled cohorts and each product (Table 2; supplemental Table 3). The time to CRS differed by product, with ide-cel having earlier time to CRS when than cilta-cel (median time to CRS of 1 day [0-1] vs 7 days [6-8]; P < .001). Of note, the median ALCmax was significantly higher for cilta-cel patients who experienced CRS than those who did not; the median ALCmax was 2.5 × 103/μL vs 0.46 × 103/μL, respectively (P = .001). Conversely, no significant difference was observed for ide-cel.
Table 2.
Univariable regression of ALCmax with other variables for all patients receiving BCMA CAR-T
| Variable | OR | 95 % CI | P value |
|---|---|---|---|
| Age at CAR-T infusion | 1.03 | 0.98-1.07 | .26 |
| Presence of CRS | |||
| No | — | — | .013 |
| Yes | 5.16 | 1.4-18.7 | |
| Highest CRS grade | |||
| Grade 1 | — | — | .016 |
| Grade 2 | 3.4 | 0.98-11.8 | |
| Grade 3 | 11.7 | 1.5-90.6 | |
| Presence of ICANS∗ | |||
| No | — | — | .004 |
| Yes | 6.7 | 1.8-24.8 | |
| High-risk CG∗ | |||
| No high-risk CG | — | — | .52 |
| High-risk present | 1.3 | 0.5-3.4 | |
| Number of previous lines | 0.9 | 0.8-1.05 | .2 |
| EMD | |||
| Nonparaskeletal EMD absent | — | — | .8 |
| Nonparaskeletal EMD | 1.1 | 0.4-3.2 | |
| ISS stage | |||
| Stage I | — | — | .2 |
| Stage II | 0.4 | 0.1-1.4 | |
| Stage III | 1.2 | 0.3-4.8 | |
| BCMA CAR-T product | |||
| Ide-cel | — | — | <.001 |
| Cilta-cel | 11.5 | 5.16-25.7 | |
The bold character to emphasize on the significant values associated ALC max and for visualization purposes.
Presence of any of deletion 17p/t(4;14)/t(14;16)/gain 1q.
ALC and outcomes after BCMA CAR-T
PFS and DoR
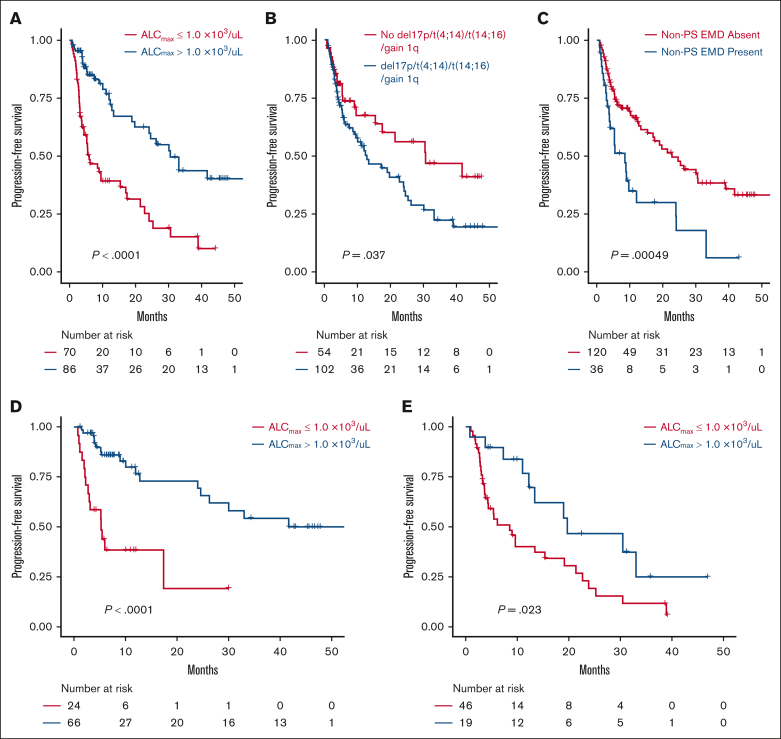
A total of 156 patients were evaluated for PFS and composed of 91 and 65 patients who received cilta-cel and ide-cel, respectively. Progression and death events were recorded in 70 and 25 patients, respectively. In univariable analysis, ALCmax >1.0 × 103/μL, ALCmax above than the median for the cohort, and ALCmax in the seconnd and third tertiles were associated with longer PFS, whereas the presence of nonparaskeletal EMD and the CAR-T product were associated with shorter PFS (supplemental Table 4A). Similar results were seen when the univariable analysis was conducted for each BCMA CAR-T product, (supplemental Table 4B). The median PFS for those with ALCmax >1.0 × 103/μL was 30.5 months (95% CI, 19.7 to not reached [NR]) vs 6.01 months (95% CI, 4.4-17.1; P < .001), with a HR of 0.34 (95% CI, 0.21-0.56; Figure 1A-C). After adjusting for the presence of nonparaskeletal EMD, high-risk cytogenetics, number of previous lines of therapy, and CAR-T product, only ALCmax >1.0 × 103/μL (HR, 0.37; 95% CI, 0.2-0.65; P < .001) and the presence of nonparaskeletal EMD (HR. 2.15; 1.3-3.5; P = .003) remained strongly associated with PFS in a multivariable Cox proportional hazard model (Table 3).
Figure 1.
Survival analysis according to ALC and other disease features. PFS by ALCmax (A), presence of high-risk cytogenetics (any of deletion 17p, t(4;14), t(14;16) or gain 1q) (B), and presence of nonparaskeletal EMD (non-PS EMD) (C) subgroups in the BCMA CAR-T cohort. PFS in patients treated with cilta-cel (D) and ide-cel (E).
Table 3.
Multivariable Cox proportional hazard model for PFS in BCMA CAR-T cohort
| Variable | HR | 95 % CI | P value |
|---|---|---|---|
| Age at CAR-T infusion | 0.99 | 0.96-1.01 | .32 |
| ALCmax, >1 × 103/μL | |||
| ALCmax, ≤1.0 × 103 cells per μL | — | — | <.001 |
| ALCmax, >1.0 × 103 cells per μL | 0.35 | 0.19-0.61 | |
| EMD | |||
| No nonparaskeletal disease | — | — | .002 |
| Nonparaskeletal EMD | 2.2 | 1.3-3.6 | |
| CAR-T product received | |||
| Ide-cel | — | — | .43 |
| Cilta-cel | 0.8 | 0.47-1.39 | |
| Number of previous lines | 1.05 | 0.99-1.1 | .1 |
| High-risk CG∗ | |||
| No high-risk CG | — | — | .061 |
| High-risk CG | 1.63 | 0.98-2.7 | |
Only patients with a follow-up >1 month were included. The bold characters emphasize the significant values associated with ALC max and for visualization purposes.
Presence of any of deletion 17p/t(4;14)/t(14;16)/gain 1q.
For DoR, similar results were seen in a cohort of 137 evaluable patients (82 cilta-cel and 55 ide-cel). In univariable analysis, ALCmax, absolute ALC change, and categorical ALCmax variables (ALCmax by tertiles, ALC above or below the median ALCmax, and ALCmax >1.0 × 103/μL) were associated with improved DoR in the pooled cohort. Factors associated with worse DoR included the presence of nonparaskeletal EMD and high-risk cytogenetics. The association of ALCmax >1.0 × 103/μL with improved DoR persisted in multivariable analysis (HR, 0.46; 95% CI, 0.2-0.9; P = .025) after controlling for nonparaskeletal EMD, high-risk cytogenetics, number of previous lines of therapy, and high-risk cytogenetics (supplemental Figure 4; supplemental Table 5)
Of note, similar results were seen when a sensitivity analysis was performed assessing the effect of receiving a lower dose than the Food and Drug Administration–approved standard-of-care dose for each product (300 × 106 to 450 × 106/kg for ide-cel and 0.5 × 106/kg to 1.0 × 106/kg for cilta-cel), the presence of out-of-specification (out-of-spec) characteristics, and the clinical trial setting (clinical trial, commercial, or as out-of-spec expanded access program). Importantly, none of these variables was significantly associated with ALCmax or PFS in univariable regression (supplemental Table 4). Additionally, when excluding patients with out-of-spec products or lower dose than the standard of care, the associations between ALC and PFS/DoR remained significant. Patient who received bendamustine (n = 10) as conditioning regimen had higher ALC at day 0 (preinfusion) than those who received flurdarabine/cyclophosphamide (n = 146; 0.09 × 103/μL [0.09 × 103 to 0.14 × 103/μL] vs 0.00 × 103/μL [0.00× 103 to 0.05 × 103/μL]; P < .001); however, there was no difference in ALCmax between the regimens (bendamustine, 1.16 × 103/μL [0.7× 103 to 1.9 × 103/μL]; flurdarabine/cyclophosphamide, 1.26 × 103/μL [0.62 × 103 to 2.82 × 103/μL]; P = .93). There was no difference in PFS by the occurrence of CRS (PFS, 19 months vs 30 months; P = .49) or ICANS (12.3 months vs 21.4 months; P = .31).
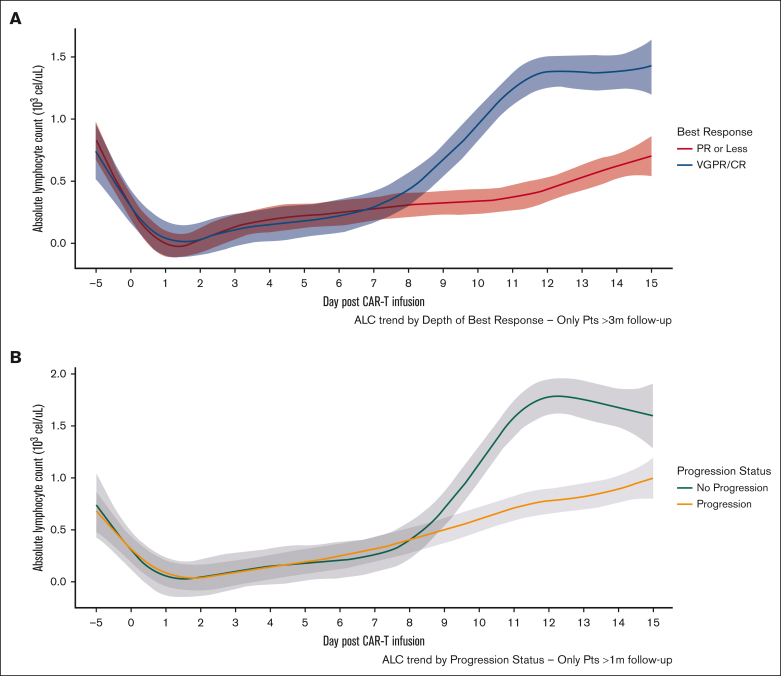
Depth of response and progression status
Patients with a very good partial response (VGPR) or CR had higher median ALCmax than those who achieved a partial response or stable disease (SD)/PD as best response (Table 4 and Figure 2A). ALCmax, absolute change in ALC, and ALCmax >1.0 × 103 cells per μL were associated with higher odds of VGPR or better in univariable logistic regression, and this effect persisted after including the presence of nonparaskeletal EMD in a multivariable regression (supplemental Table 6). Importantly, preconditioning ALC and baseline ALC had no impact on depth of response, and there was no relationship in terms of time to initial or best response with ALCmax. The presence of CRS was associated with VGPR or better (83% vs 44%; P < .001); however, there were no differences in terms of depth of response by the occurrence of ICANS (69% vs 80%; P = .34).
Table 4.
Depth of response and progression status at last follow-up
| Best response |
All patients |
|||
|---|---|---|---|---|
| VGPR/CR n = 113 |
PR n = 14 |
SD/PD n = 17 |
P value | |
| Median ALCmax, ×103/μL (IQR) |
1.5 (0.8-3.1) |
0.8 (0.5-1.3) |
0.5 (0.4-0.7) |
<.001 |
|
Progression status |
Nonprogressor n = 88 |
Progressor n = 68 |
P value |
|
| Median ALCmax, ×103/μL (IQR) | 1.82 (0.92-4.27) | 0.79 (0.49-1.40) | <.001 | |
Only patients with >3 months of follow-up were included in the best response assessment. Response and progression based on International Myeloma Working Group criteria.
Figure 2.
Comparison of ALC kinetics according to clinical outcomes. ALC by depth of response status (A) and progression status (B) from days –5 through +15. Shaded area represents 95% CI.
Additionally, when the occurrence of disease progression was assessed as a dichotomous variable in patients with >6 month of follow-up, those who had not experienced disease progression had a higher median ALCmax than those who had progressed (1.86 × 103/μL vs 0.79 × 103/μL, respectively; P < .001). Similarly, patients who had achieved VGPR or better response had higher ALCmax (1.67 × 103/μL vs 0.5 × 103/μL; P < .001) (Figure 2B). Of note, these findings were seen when analyzing each product alone (supplemental Figure 5). Furthermore, an ALCmax >1 × 103/μL was associated with lesser odds of progression on univariate logistic regression for the whole cohort and for each product as well (supplemental Table 7) and remained significant when including nonparaskeletal EMD in multivariable analysis (supplemental Table 8).
Specific ALCmax cutoff as prognostic tools
Given the strong association of ALCmax with depth of response and PFS, we assessed whether different ALCmax cutoffs as set thresholds would be clinically applicable to identify patients with the likelihood of deeper response or improved PFS or those with high-risk of lack of response or early progression.
An ALCmax ≤0.5 × 103/μL identified patients with lack of response and shorter PFS due to progression on follow-up
Patients with a ALCmax ≤0.5 × 103/μL during days 0 through +15 represented a group with poor outcomes, regardless of the BCMA CAR-T product received. Patients with ALCmax ≤0.5 × 103/μL had a lower median PFS than those with ALCmax >0.5 × 103/μL (3.7 [95% CI, 2.5 to NR] vs 24.1 months [95% CI, 17.4-24.1], respectively; P < .001). Similar results were seen when this cutoff was assessed individually in each BCMA CAR-T product, with a median PFS of 2.1 (95% CI, 1.6 to NR) vs 30.1 months (95% CI, 12.8 to NR; P = .004) for patients who received cilta-cel and 4.4 months (95% CI, 2.8 to NR) vs 19 months (95% CI, 9.6-33.1; P = .002) for patients treated with ide-cel (Figure 1).
Patients with ALCmax >1.0 × 103/μL had improved PFS
Similarly, patients with a ALCmax >1.0 × 103/μL had longer PFS than those with a lower ALCmax after CAR-T infusion (33.1 months [95% CI, 24.1 to NR] vs 6 months [95% CI, 4.4-15.4]; P < .001). This was also seen in both products, with patients who received ide-cel (19.8 months vs 8.5 months; P = .023) or cilta-cel (NR vs 5.3 months; P < .0001) having a better PFS (Figure 1D-E).
As mentioned before, an ALCmax of >1.0 × 103/μL was associated with improved PFS in univariable regression analysis and after adjusting for CAR-T product, presence of EMD, number of treatment lines, and presence of high-risk cytogenetics in multivariable Cox model. Furthermore, an ALCmax >1.0 × 103/μL remained associated with improved PFS when applied to patients with high-risk cytogenetics (19.8 months vs 5.3 months; P < .001) and nonparaskeletal EMD (12 months vs 4.5 months; P = .008; Table 3; supplemental Figures 4-6).
Baseline characteristics by ALCmax >1 × 103/μL are shown on supplemental Table 9. Higher rates of cytotoxic chemotherapy, bendamustine, and cyclophosphamide use as part of last line before apheresis were seen in the group with ALCmax <1.0 × 103/μL, as well as lower ALC and higher D-dimer prelymphodepletion. There was no difference in ferritin, C-reactive protein, or D-dimer at baseline or highest ferritin during first 30 days after infusion between those with ALCmax ≤1.0 × 103/μL and >1 × 103/μL. Furthermore, highest ferritin during first 30 days was the only inflammatory biomarker that had any correlation with ALCmax; however, the correlation was weak (Spearman r = 0.23; P = .003).
Immune effector cell hematological toxicity and ALC
When assessed from days 30 through 60, a total of 70 (52.2%), 93 (67.4%), and 19 patients (13.7%) developed any neutropenia, thrombocytopenia, and anemia, respectively. There were no differences in terms of median ALCmax in those patients with cytopenia with those without the respective cytopenia, either when all cytopenia grades were pooled or each specific grade compared with the noncytopenia group (supplemental Figure 7).
Infectious events and ALC
Of 156 patients, 33 (21.1%) had nonneutropenic infectious events during the first 30 days after CAR-T. There were no differences in ALCmax between those who had an infectious episode and those who did not (1.4 × 103/μL [0.8× 103 to 3.4 × 103/μL] vs 1.25 × 103/μL [0.6 × 103/μL to 2.5 × 103/μL]; P = .2, respectively). Furthermore, there were no differences in terms of maximal ANC during days 0 to 15, ALCmax >1.0 ×103/μL, age at CAR-T infusion, or the year of CAR-T administration. Characteristics of infectious events and the use of Intravenous immunoglobulins (IVIG), ANC, and ALC at the time of infection can be seen in supplemental Tables 10 and 11.
Lymphocytosis after BCMA CAR-T is mainly driven by expansion of BCMA CAR-T lymphocytes
Peripheral blood was collected for flow cytometry in 4 patients on days 7, 14, and 21. The ALCmax values were 4.3 × 103/μL, 13.7 × 103/μL, 2.2 × 103/μL, and 0.4 × 103/μL and occurred at day 13 for all 4 patients. Flow cytometry showed presence of BCMA CAR-T lymphocytes in all patients on day 7 at low quantities, representing <0.2% of the T lymphocytes. This was followed by significant expansion of the total lymphocytes and BCMA CAR-T on day 14, with BCMA CAR-T representing 54% to 57% of T lymphocytes in 2 patients and 88.6% in the third (supplemental Figure 8A-C). At day 21, there was persistence of BCMA CAR-T in all 3 cases, although with lower proportion of BCMA CAR-T lymphocytes, representing 40.05%, 66.7%, and 48.8% of T cells (supplemental Figure 9). These 3 patients responded to BCMA CAR-T without disease progression at the latest follow-up. However, in the fourth patient who had an ALCmax of 0.4 × 103/μL and SD that subsequently progressed within 2 months of CAR-T infusion, the BCMA CAR-T represented only 1% and 0.2% of T lymphocytes on days 14 and 21, respectively (supplemental Figure 8D). We observed a significant strong correlation between ALC and the percentage of BCMA positive T cells (Spearman r = 0.93; P < .0001), suggesting that ALC is a good indicator for CAR-T expansion.
ALC dynamics differ from BCMA CAR-T and CD19 CAR-T for NHL
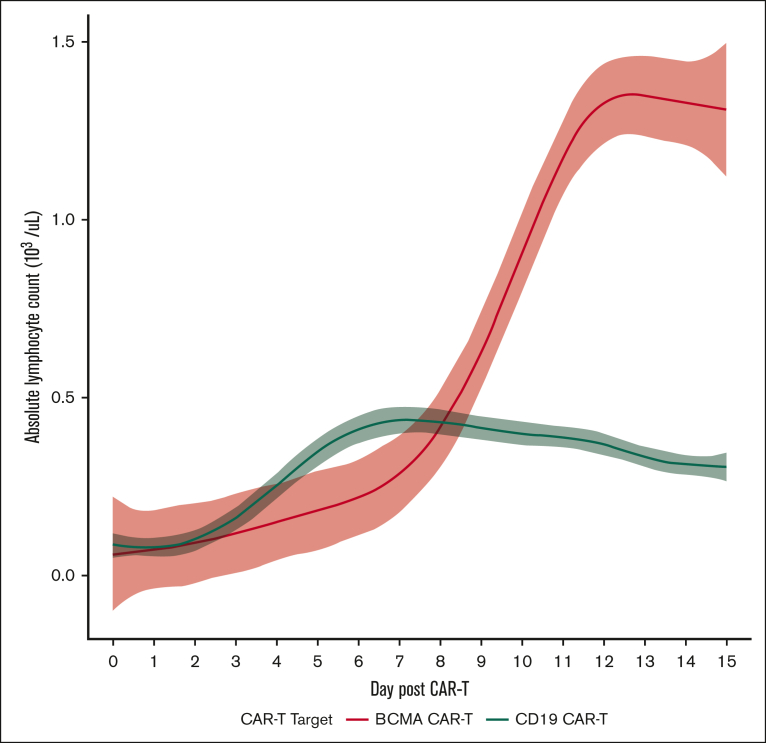
The ALC dynamics during days –5 through +15 between patients receiving BCMA CAR-T and a cohort of patients receiving CD19 CAR-T for NHL at Weill Cornell Medicine were compared. A total of 105 patients with NHL received CD19 CAR-T (82 patients received axicabtagene ciloleucel, 7 patients received brexucabtagene autoleucel, 7 patients tisagenlecleucel, and 9 received lisocabtagene maraleucel). The main indications for CD19 CAR-T included R/R NHL (81 patients for large B-cell lymphoma, 7 patients for follicular lymphoma, 4 patients for marginal zone lymphoma, and 1 patient for small lymphocytic lymphoma, and 7 patients for mantle cell lymphoma). The ALC dynamics varied when comparing both BCMA vs CD19 CAR-T and by individual product. The median ALCmax for BCMA CAR-T was 1.3 × 103/μL (0.7 × 103 to 2.9 × 103/μL), with a median time to ALCmax of 12 days (10-13), whereas the median ALCmax for CD19 CAR-T was 0.47 × 103/μL (0.3 × 103 to 0.8 × 103/μL), with a median time to ALCmax of 10 days (8-13), which were significantly different (P < .001 for both; Figure 3). Of note, there were no differences in ALC on day 0 before infusion between BCMA CAR-T and CD19 CAR-T. There was no correlation of best response after CD19 CAR-T as assessed by the Lugano response criteria with ALCmax, with no differences in median ALCmax across overall response or depth of response (median ALCmax for CR, 0.5 ×103/μL [0.3 × 103 to 0.8 × 103/μL]; partial response, 0.40 ×103/μL [0.2 × 103 to 0.75 × 103/μL]; SD/PD, 0.7 ×103/μL [0.4 × 103/μL to 1 × 103/μL]; P = .11).
Figure 3.
ALC dynamics in BCMA and CD19 CAR-T–treated patients during days 0 through +15.
Discussion
Although CAR-T expansion after infusion is a well-described phenomenon across different hematological malignancies and CAR-T constructs, overt lymphocytosis in the setting of recent lymphodepleting conditioning has not been widely investigated, and this phenomenon had not been reported for the cases of CD19 CAR-T for NHL or BCMA CAR-T for MM.
In this study of 156 patients with R/R MM undergoing BMCA CAR-T with either cilta-cel or ide-cel, baseline ALC after lymphodepleting chemotherapy was similar. We observed an increase in post–CAR-T ALC with both products, with a median ALCmax of 1.26 × 103/μL across the cohort. Furthermore, differences in ALC kinetics, both in terms of ALCmax and time to ALCmax, were identified by product, with higher ALCmax peaking at a later time point for cilta-cel than ide-cel. The occurrence of CRS and ICANS preceded ALCmax and were directly correlated with ALC expansion after BCMA CAR-T infusion.
Importantly, the degree of ALC increase, either measured by ALCmax or absolute ALC change from baseline, were associated with improved relevant clinical outcomes such as response rate, depth of response, and importantly PFS and DoR. Notably, the association between higher ALCmax and PFS/DoR was maintained after controlling for age, the presence of nonparaskeletal EMD, high-risk cytogenetics, number of previous lines, tumor burden, inflammatory biomarkers, and CAR-T product. Furthermore, an ALCmax cutoff point of ≤0.5 × 103/μL identified a population of patients with poor outcomes, with a median PFS <4 months irrespective of BCMA CAR-T product used. Conversely, patients with ALCmax >1.0 × 103/μL had significantly better deeper responses and a median PFS of 30 months. Based on these findings, we believe these 2 cutoffs represent useful clinical prognostic indicators that could be easily measured early after CAR-T infusion.
Our analysis of this large multicenter study showed that last line alkylators before leukapheresis, and specifically bendamustine, were associated with a significantly lower ALCmax and worse PFS. This observation is consistent with previous findings in patients with large B-cell lymphoma.17 Although we did not perform profiling of relevant cytokines, we observed that baseline inflammatory markers such as ferritin, C-reactive protein, and D-dimer were associated with worse outcomes. However, in multivariate analysis, only ALCmax >1.0 × 103/μL and nonparaskeletal EMD were the only independent factors for PFS. Analysis of different groups by CAR-T product or ALCmax levels showed no significant differences in terms of pre–CAR-T tumor burden, high-risk cytogenetics, presence of nonparaskeletal EMD, or previous lines between the 2 products or ALCmax groups; however, higher proportion of patients in the group with lower ALCmax (≤1 × 103/uL) received bendamustine before CAR-T, had lower median prelymphodepletion ALC, and had higher median D-dimer at baseline. These findings suggest that preinfusion patient characteristics such as exposure to alkylating agents or inflammatory status may also contribute to response to CAR-T and CAR-T expansion as evidenced by ALC.
The link between ALC and CAR-T expansion was confirmed in 4 patients. Flow Cytometry analysis at days 7, 14, and 21 showed that ALCmax was specifically driven by BCMA CAR-T lymphocytes, with overall kinetics for the CAR-Ts reflecting those seen in ALC, suggesting that it could be a widely accessible surrogate to assess BCMA CAR-T expansion in clinical practice. Recently, a study on 27 patients with R/R MM receiving ide-cel showed that expansion of CAR-Ts was associated with better outcomes.18 Although BCMA CAR-T expansion in our study is responsible for most of the observed increase in ALC, non–CAR-T lymphocytes are also part of the increase in ALC, suggesting that expansion of endogenous or non–CAR-T lymphocytes also plays a role. Nonetheless, future studies that elucidate the differences in T-cell phenotypes of both CAR and native T cells are needed to understand this phenomenon and how it affects different outcomes. Moreover, although a higher ALCmax during days 0 through +15 is highly predictive of response, long-term progression may be influenced by additional factors such as CAR-T persistence, exhaustion, or disease-specific characteristics leading to tumor escape and resistance.
In previous study of patients with B-ALL receiving CD19 CAR-T, high ALC was frequent and correlated with depth of response and outcomes; however, we did not observe this relationship between ALCmax and response in NHL in our cohort. Furthermore, clear differences in terms of ALC kinetics were evident between BCMA and 4 CD19 CAR-T products, suggesting that post–CAR-T lymphocytosis may be influenced by disease-specific characteristics as seen between BCMA CAR-T for MM and CD19 CAR-T for NHL and B-ALL.
In conclusion, our study presents ALC in the first 15 days after infusion as a novel, yet widely available, biomarker for predicting response to BCMA CAR-T therapy in the R/R MM setting. We propose an ALCmax ≤0.5 × 103/μL as a negative prognostic factor associated with worse responses and shorter PFS and ALCmax >1.0 × 103/μL as a positive prognostic factor that portends deeper and more durable response to BCMA CAR-T therapy. Our findings also open the door for further understanding of the phenotypic differences in the immune microenvironment, CAR-T characteristics, and T-cell characteristics between patients with higher ALC and those with low levels.
Conflict-of-interest disclosure: J.M. reports receiving advisory or consulting fees from Janssen. C.R. reports receiving research funding from Janssen. D.P. reports receiving consulting fees from Sanofi. M.M. reports receiving advisory or consulting fees from bluebird bio, CRISPER/Vertex, and Incyte. S.L. reports receiving advisory or consulting fees from Adaptive Biotechnologies, Alexion Pharmaceuticals, Bristol Myers Squibb, Janssen, Karyopharm Therapeutics, Takeda, Pfizer, Oncopeptides, and Caelum Biosciences, and patents and royalties effective 1 January 2041; research funding from Celgene and Sanofi; and honoraria from Clinical Care Options and Regeneron. R.R. reports receiving advisory or consulting fees from TScan Therapeutics. S.Y. reports receiving advisory and consultancy fees from Kite Pharma and Bristol Myers Squibb. A.R. reports receiving advisory or consulting fees from Sanofi, Bristol Myers Squibb, and Adaptive Biotechnologies. S.P. reports receiving research funding from Amgen, Karyopharm, and Bristol Myers Squibb. S.J. reports receiving advisory or consulting fees from Bristol Myers Squibb, Janssen, Merck, Sanofi, Takeda, and Karyopharm. R.N. received honoraria and advisory fees from Bristol Myers Squib, Janssen, and Karyopharm, and received research funding from Celgene Inc, Takeda Inc, and Onyx Inc. S.R. reports receiving advisory or consulting fees from Bristol Myers Squibb and Janssen; and research support from Janssen, Bristol Myers Squibb, Gracell Biotechnologies, and C4 Therapeutics. M.B. reports receiving honoraria from Bristol Myers Squibb, Janssen, and Karyopharm; and consultancy fees from Bristol Myers Squibb, Janssen, Ipsen, and Menarini Silicon Biosystems. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank all patients who participated in the study and their caregivers.
This work was supported in part by Weill Cornell Medicine Funds for the Future grant (M.B.) and American Society of Clinical Oncology (ASCO) Conquer Cancer Young Investigator award (M.M.S. and M.B.).
Authorship
Contribution: M.M.S. and M.B. formulated and designed the study, coordinated clinical and sample collection, data analysis and interpretation, and wrote the manuscript; D.P. participated in clinical data collection, study design, and data analysis; C.U. and T.H.M. contributed with data collection and manuscript elaboration; J.A.F., C.G., H.T.C., J.E.V.-H., K.E., and S.T. contributed with data collection; J.M., C.R., and R.P. contributed with sample collection and study concept; S.Y. contributed with study concept and data interpretation; G.I. contributed with patient sample processing, flow cytometry, and data interpretation; D.J. contributed with patient sample collection; M.M., S.L., R.R., A.R., and S.J. contributed with data interpretation; S.R. and R.N. contributed with study design, data interpretation, and provided logistical support for study completion; M.B. contributed with study design, data collection, analysis, interpretation of results, and manuscript elaboration; and all authors reviewed and contributed to final manuscript elaboration.
Footnotes
M.M.S. and D.P. are joint first authors.
S.R., R.N., and M.B. are joint senior authors.
Data are available on request from the corresponding author, Mark Bustoros (mab4033@med.cornell.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Rodriguez-Otero P, Ailawadhi S, Arnulf B, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388(11):1002–1014. doi: 10.1056/NEJMoa2213614. [DOI] [PubMed] [Google Scholar]
- 2.San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. 2023;389(4):335–347. doi: 10.1056/NEJMoa2303379. [DOI] [PubMed] [Google Scholar]
- 3.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 4.Munshi NC, Anderson LD, Jr., Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 5.Gagelmann N, Ayuk FA, Klyuchnikov E, Wolschke C, Berger SC, Kröger N. Impact of high-risk disease on the efficacy of chimeric antigen receptor T-cell therapy for multiple myeloma: a meta-analysis of 723 patients. Haematologica. 2023;108(10):2799–2802. doi: 10.3324/haematol.2022.282510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Liu M, Yuan T, Yan L, Cui R, Deng Q. Efficacy and follow-up of humanized anti-BCMA CAR-T cell therapy in relapsed/refractory multiple myeloma patients with extramedullary-extraosseous, extramedullary-bone related, and without extramedullary disease. Hematol Oncol. 2022;40(2):223–232. doi: 10.1002/hon.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal R, Baksh M, Larsen JT, et al. Prognostic value of early bone marrow MRD status in CAR-T therapy for myeloma. Blood Cancer J. 2023;13(1):1–5. doi: 10.1038/s41408-023-00820-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bove C, Arcangeli S, Falcone L, et al. CD4 CAR-T cells targeting CD19 play a key role in exacerbating cytokine release syndrome, while maintaining long-term responses. J Immunother Cancer. 2023;11(1):e005878. doi: 10.1136/jitc-2022-005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melenhorst JJ, Chen GM, Wang M, et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature. 2022;602(7897):503–509. doi: 10.1038/s41586-021-04390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faude S, Wei J, Muralidharan K, et al. Absolute lymphocyte count proliferation kinetics after CAR T-cell infusion impact response and relapse. Blood Adv. 2021;5(8):2128–2136. doi: 10.1182/bloodadvances.2020004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 13.Paiva B, Manrique I, Rytlewski J, et al. Time-dependent prognostic value of serological and measurable residual disease assessments after idecabtagene vicleucel. Blood Cancer Discov. 2023;4(5):365–373. doi: 10.1158/2643-3230.BCD-23-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 15.Rejeski K, Subklewe M, Aljurf M, et al. Immune effector cell–associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. 2023;142(10):865–877. doi: 10.1182/blood.2023020578. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacoboni G, Navarro V, Martín-López AÁ, et al. Recent bendamustine treatment before apheresis has a negative impact on outcomes in patients with large B-cell lymphoma receiving chimeric antigen receptor T-cell therapy. J Clin Oncol. 2024;42(2):205–217. doi: 10.1200/JCO.23.01097. [DOI] [PubMed] [Google Scholar]
- 18.Fischer L, Grieb N, Born P, et al. Cellular dynamics following CAR T cell therapy are associated with response and toxicity in relapsed/refractory myeloma. Leukemia. 2024;38(2):372–382. doi: 10.1038/s41375-023-02129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.