Abstract
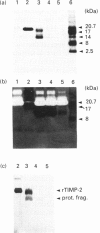
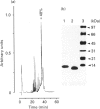
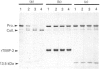
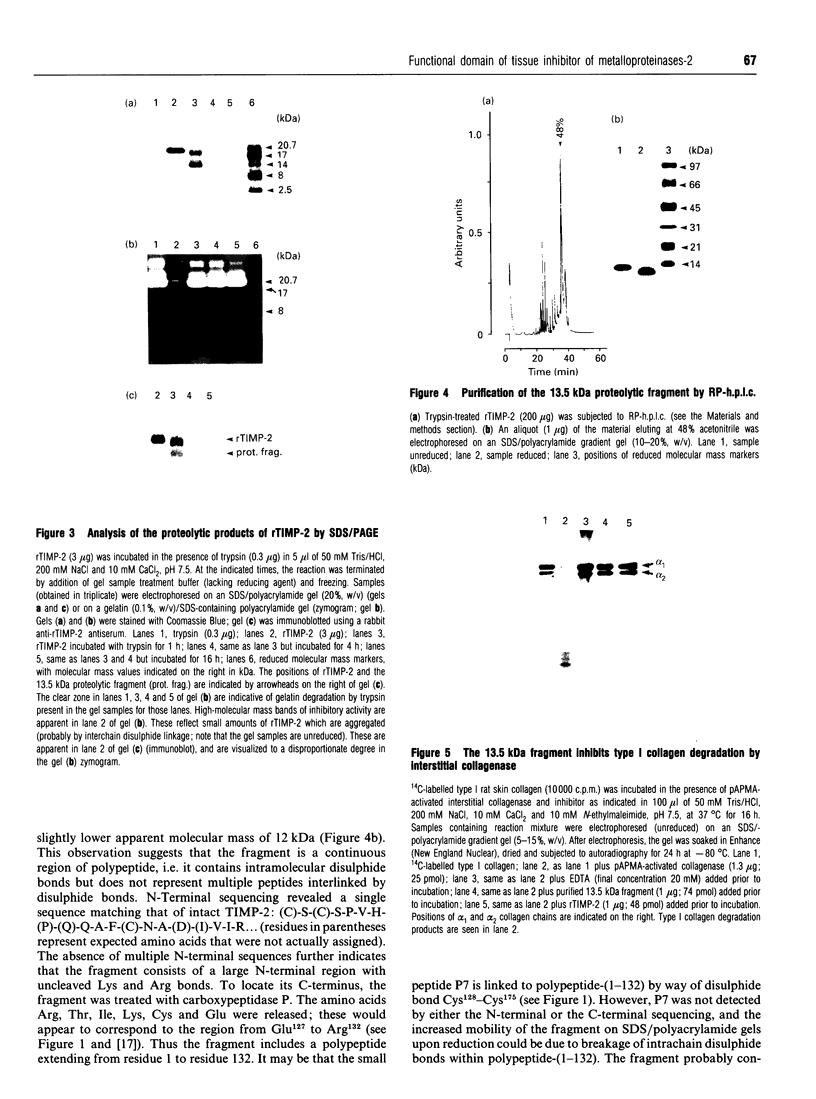
Analysis of the functional domain of tissue inhibitor of metallo-proteinases-2 (TIMP-2) was performed using limited proteolytic degradation with trypsin. This treatment generated a 13.5 kDa fragment which was purified and shown to consist of an uncleaved N-terminal region extending from residue 1 to residue 132. The fragment retains the ability to inhibit activated interstitial collagenase and to block the autocatalytic activation of procollagenase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone T. C., Johnson M. J., De Clerck Y. A., Langley K. E. cDNA cloning and expression of a metalloproteinase inhibitor related to tissue inhibitor of metalloproteinases. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2800–2804. doi: 10.1073/pnas.87.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carmichael D. F., Sommer A., Thompson R. C., Anderson D. C., Smith C. G., Welgus H. G., Stricklin G. P. Primary structure and cDNA cloning of human fibroblast collagenase inhibitor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2407–2411. doi: 10.1073/pnas.83.8.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Galloway W. A., Mercer E., Murphy G., Reynolds J. J. Purification of rabbit bone inhibitor of collagenase. Biochem J. 1981 Apr 1;195(1):159–165. doi: 10.1042/bj1950159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. M., Cawston T. E. Fragments of human fibroblast collagenase. Purification and characterization. Biochem J. 1989 Oct 1;263(1):201–206. doi: 10.1042/bj2630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe B., Skup D. In vitro synthesis of the active tissue inhibitor of metalloproteinases encoded by a complementary DNA from virus-infected murine fibroblasts. J Biol Chem. 1988 Jan 25;263(3):1439–1443. [PubMed] [Google Scholar]
- De Clerck Y. A., Yean T. D., Ratzkin B. J., Lu H. S., Langley K. E. Purification and characterization of two related but distinct metalloproteinase inhibitors secreted by bovine aortic endothelial cells. J Biol Chem. 1989 Oct 15;264(29):17445–17453. [PubMed] [Google Scholar]
- DeClerck Y. A. Purification and characterization of a collagenase inhibitor produced by bovine vascular smooth muscle cells. Arch Biochem Biophys. 1988 Aug 15;265(1):28–37. doi: 10.1016/0003-9861(88)90367-0. [DOI] [PubMed] [Google Scholar]
- DeClerck Y. A., Yean T. D., Lu H. S., Ting J., Langley K. E. Inhibition of autoproteolytic activation of interstitial procollagenase by recombinant metalloproteinase inhibitor MI/TIMP-2. J Biol Chem. 1991 Feb 25;266(6):3893–3899. [PubMed] [Google Scholar]
- Dean D. D., Woessner J. F., Jr Extracts of human articular cartilage contain an inhibitor of tissue metalloproteinases. Biochem J. 1984 Feb 15;218(1):277–280. doi: 10.1042/bj2180277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Marmer B. L., Grant G. A., Eisen A. Z., Wilhelm S., He C. S. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8207–8211. doi: 10.1073/pnas.86.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Johnson-Wint B. A quantitative collagen film collagenase assay for large numbers of samples. Anal Biochem. 1980 May 1;104(1):175–181. doi: 10.1016/0003-2697(80)90295-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Reynolds J. J. An inhibitor of collagenase from human amniotic fluid. Purification, characterization and action on metalloproteinases. Biochem J. 1981 Apr 1;195(1):167–170. doi: 10.1042/bj1950167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Houbrechts A., Cockett M. I., Williamson R. A., O'Shea M., Docherty A. J. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991 Aug 20;30(33):8097–8102. doi: 10.1021/bi00247a001. [DOI] [PubMed] [Google Scholar]
- Okada Y., Watanabe S., Nakanishi I., Kishi J., Hayakawa T., Watorek W., Travis J., Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988 Feb 29;229(1):157–160. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- Springman E. B., Angleton E. L., Birkedal-Hansen H., Van Wart H. E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a "cysteine switch" mechanism for activation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Brown P. D., Onisto M., Levy A. T., Liotta L. A. Tissue inhibitor of metalloproteinases-2 (TIMP-2) mRNA expression in tumor cell lines and human tumor tissues. J Biol Chem. 1990 Aug 15;265(23):13933–13938. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Stricklin G. P., Welgus H. G. Human skin fibroblast collagenase inhibitor. Purification and biochemical characterization. J Biol Chem. 1983 Oct 25;258(20):12252–12258. [PubMed] [Google Scholar]
- Ward R. V., Atkinson S. J., Slocombe P. M., Docherty A. J., Reynolds J. J., Murphy G. Tissue inhibitor of metalloproteinases-2 inhibits the activation of 72 kDa progelatinase by fibroblast membranes. Biochim Biophys Acta. 1991 Aug 30;1079(2):242–246. doi: 10.1016/0167-4838(91)90132-j. [DOI] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Williamson R. A., Marston F. A., Angal S., Koklitis P., Panico M., Morris H. R., Carne A. F., Smith B. J., Harris T. J., Freedman R. B. Disulphide bond assignment in human tissue inhibitor of metalloproteinases (TIMP). Biochem J. 1990 Jun 1;268(2):267–274. doi: 10.1042/bj2680267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]