Abstract
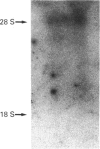
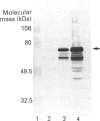
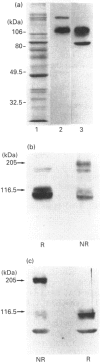
We examined the protein and mRNA encoding the amiloride-sensitive Na+/H+ exchanger from human placenta. Reverse transcriptase PCR of human placental RNA and a human choriocarcinoma cell line showed that the message for the amiloride-sensitive Na+/H+ exchanger from human placenta. Reverse transcriptase PCR of human placental RNA and a human choriocarcinoma cell line showed that the message for the amiloride-sensitive Na+/H+ exchanger is present in the placenta and its derived cell line. Northern blot analysis showed only one species of Na+/H+ exchanger mRNA, of about 5 kb in size. To examine the Na+/H+ exchanger protein two different affinity-purified antibodies were produced against the C-terminal cytoplasmic region of the Na+/H+ exchanger. The antibodies both identified a 105 kDa protein in human placental brush border membrane vesicles. Under non-reducing conditions the amount of 105 kDa protein was greatly decreased, while a 205 kDa protein became apparent. This is probably a dimer of the 105 kDa protein. The monomer-to-dimer transition was dependent on the concentration of beta-mercaptoethanol. The results show that the amiloride-sensitive Na+/H+ exchanger is relatively abundant in human placenta and that it can exist as a larger 205 kDa protein linked by disulphide bonds.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkovetz D. F., Leibach F. H., Mahesh V. B., Devoe L. D., Cragoe E. J., Jr, Ganapathy V. Na+-H+ exchanger of human placental brush-border membrane: identification and characterization. Am J Physiol. 1986 Dec;251(6 Pt 1):C852–C860. doi: 10.1152/ajpcell.1986.251.6.C852. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Béliveau R., Demeule M., Potier M. Molecular size of the Na+-H+ antiport in renal brush border membranes, as estimated by radiation inactivation. Biochem Biophys Res Commun. 1988 Apr 15;152(1):484–489. doi: 10.1016/s0006-291x(88)80739-3. [DOI] [PubMed] [Google Scholar]
- Casavola V., Helmle-Kolb C., Murer H. Separate regulatory control of apical and basolateral Na+/H+ exchange in renal epithelial cells. Biochem Biophys Res Commun. 1989 Dec 15;165(2):833–837. doi: 10.1016/s0006-291x(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976 Nov;262(3):755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L., Leberer E., Green N. M., MacLennan D. H. The fast-twitch muscle calsequestrin isoform predominates in rabbit slow-twitch soleus muscle. FEBS Lett. 1989 Jan 2;242(2):297–300. doi: 10.1016/0014-5793(89)80488-0. [DOI] [PubMed] [Google Scholar]
- Fliegel L., Sardet C., Pouyssegur J., Barr A. Identification of the protein and cDNA of the cardiac Na+/H+ exchanger. FEBS Lett. 1991 Feb 11;279(1):25–29. doi: 10.1016/0014-5793(91)80241-t. [DOI] [PubMed] [Google Scholar]
- Friedrich T., Sablotni J., Burckhardt G. Identification of the renal Na+/H+ exchanger with N,N'-dicyclohexylcarbodiimide (DCCD) and amiloride analogues. J Membr Biol. 1986;94(3):253–266. doi: 10.1007/BF01869721. [DOI] [PubMed] [Google Scholar]
- Haggerty J. G., Agarwal N., Reilly R. F., Adelberg E. A., Slayman C. W. Pharmacologically different Na/H antiporters on the apical and basolateral surfaces of cultured porcine kidney cells (LLC-PK1). Proc Natl Acad Sci U S A. 1988 Sep;85(18):6797–6801. doi: 10.1073/pnas.85.18.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F., Pizzonia J. H., Reilly R. F., Rebouças N. A., Sardet C., Pouysségur J., Slayman C. W., Aronson P. S., Igarashi P. Cloning, sequence, and tissue distribution of a rabbit renal Na+/H+ exchanger transcript. Biochim Biophys Acta. 1991 Dec 2;1129(1):105–108. doi: 10.1016/0167-4781(91)90221-7. [DOI] [PubMed] [Google Scholar]
- Igarashi P., Aronson P. S. Covalent modification of the renal Na+/H+ exchanger by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1987 Jan 15;262(2):860–868. [PubMed] [Google Scholar]
- Kulanthaivel P., Leibach F. H., Mahesh V. B., Cragoe E. J., Jr, Ganapathy V. The Na(+)-H+ exchanger of the placental brush-border membrane is pharmacologically distinct from that of the renal brush-border membrane. J Biol Chem. 1990 Jan 25;265(3):1249–1252. [PubMed] [Google Scholar]
- Kulanthaivel P., Simon B. J., Leibach F. H., Mahesh V. B., Ganapathy V. An essential role for vicinal dithiol groups in the catalytic activity of the human placental Na(+)-H+ exchanger. Biochim Biophys Acta. 1990 May 24;1024(2):385–389. doi: 10.1016/0005-2736(90)90369-y. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahnensmith R. L., Aronson P. S. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985 Jun;56(6):773–788. doi: 10.1161/01.res.56.6.773. [DOI] [PubMed] [Google Scholar]
- Michalak M., Fliegel L., Wlasichuk K. Isolation and characterization of calcium binding glycoproteins of cardiac sarcolemmal vesicles. J Biol Chem. 1990 Apr 5;265(10):5869–5874. [PubMed] [Google Scholar]
- Orlowski J., Kandasamy R. A., Shull G. E. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992 May 5;267(13):9331–9339. [PubMed] [Google Scholar]
- Otsu K., Kinsella J., Sacktor B., Froehlich J. P. Transient state kinetic evidence for an oligomer in the mechanism of Na+-H+ exchange. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4818–4822. doi: 10.1073/pnas.86.13.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter T., King S. M., Witman G. B. A two-step procedure for efficient electrotransfer of both high-molecular-weight (greater than 400,000) and low-molecular-weight (less than 20,000) proteins. Anal Biochem. 1987 May 1;162(2):370–377. doi: 10.1016/0003-2697(87)90406-4. [DOI] [PubMed] [Google Scholar]
- Pattillo R. A., Gey G. O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968 Jul;28(7):1231–1236. [PubMed] [Google Scholar]
- Ross W., Bertrand W., Morrison A. A photoactivatable probe for the Na+/H+ exchanger cross-links a 66-kDa renal brush border membrane protein. J Biol Chem. 1990 Apr 5;265(10):5341–5344. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sardet C., Counillon L., Franchi A., Pouysségur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990 Feb 9;247(4943):723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Speeg K. V., Jr, Azizkhan J. C., Stromberg K. The stimulation by methotrexate of human chorionic gonadotropin and placental alkaline phosphatase in cultured choriocarcinoma cells. Cancer Res. 1976 Dec;36(12):4570–4576. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway J. L., Morrison B. D., Wemmie J. A., Frias I., O'Hare T., Pilch P. F., Pessin J. E. The endogenous functional turkey erythrocyte and rat liver insulin receptor is an alpha 2 beta 2 heterotetrameric complex. Biochem J. 1990 Oct 1;271(1):99–105. doi: 10.1042/bj2710099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Fanger B. O., Guyer C. A., Staros J. V. Electrophoretic transfer of high-molecular-weight proteins for immunostaining. Methods Enzymol. 1989;172:687–696. doi: 10.1016/s0076-6879(89)72038-3. [DOI] [PubMed] [Google Scholar]
- Wu J. S., Lever J. E. Photoaffinity labeling by [3H]-N5-methyl-N5-isobutylamiloride of proteins which cofractionate with Na+/H+ antiport activity. Biochemistry. 1989 Apr 4;28(7):2980–2984. doi: 10.1021/bi00433a036. [DOI] [PubMed] [Google Scholar]