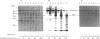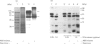Abstract
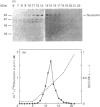
Nucleolin is a major nucleolar phosphoprotein and is presumably involved in rDNA transcription and ribosome biosynthesis. This protein is known to be very labile and to be cleaved by endogenous proteases into many small peptides. We found that, when rat liver nucleolar suspension (Nu-1) or nucleolin-rich extract (Nu-2) was incubated under conventional conditions, polyamines and histones interacted with the nucleolin to lead to its preferential degradation to 60 kDa phosphopeptide (p60). The peptide p60 was identified as a peptide containing the N-terminal half of the nucleolin molecule, as judged from peptide-map analysis. Whereas spermine binding to the purified nucleolin was decreased by KCl concentrations above 50 mM, histones (H1, H2B and H3) were able to bind to the nucleolin in the presence of up to 300 mM KCl. A distinct difference between H1 and other histones was found in that H1 could produce p60 from nucleolin in both Nu-1 and Nu-2, whereas H2B and H3 stimulated the degradation of nucleolin to p60 only when Nu-2 was used for the source of nucleolin. A possible relationship between p60 formation and rRNA synthesis is discussed, but its exact role remains to be studied.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenguer P., Baldin V., Mathieu C., Prats H., Bensaid M., Bouche G., Amalric F. Protein kinase NII and the regulation of rDNA transcription in mammalian cells. Nucleic Acids Res. 1989 Aug 25;17(16):6625–6636. doi: 10.1093/nar/17.16.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenguer P., Caizergues-Ferrer M., Labbé J. C., Dorée M., Amalric F. Mitosis-specific phosphorylation of nucleolin by p34cdc2 protein kinase. Mol Cell Biol. 1990 Jul;10(7):3607–3618. doi: 10.1128/mcb.10.7.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G., Caizergues-Ferrer M., Bugler B., Amalric F. Interrelations between the maturation of a 100 kDa nucleolar protein and pre rRNA synthesis in CHO cells. Nucleic Acids Res. 1984 Apr 11;12(7):3025–3035. doi: 10.1093/nar/12.7.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon H. M., Amalric F. Nucleolin gene organization in rodents: highly conserved sequences within three of the 13 introns. Gene. 1990 Apr 16;88(2):187–196. doi: 10.1016/0378-1119(90)90031-l. [DOI] [PubMed] [Google Scholar]
- Bourbon H. M., Bugler B., Caizergues-Ferrer M., Amalric F., Zalta J. P. Maturation of a 100 kDa protein associated with preribosomes in Chinese hamster ovary cells. Mol Biol Rep. 1983 May;9(1-2):39–47. doi: 10.1007/BF00777472. [DOI] [PubMed] [Google Scholar]
- Bourbon H. M., Lapeyre B., Amalric F. Structure of the mouse nucleolin gene. The complete sequence reveals that each RNA binding domain is encoded by two independent exons. J Mol Biol. 1988 Apr 20;200(4):627–638. doi: 10.1016/0022-2836(88)90476-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bugler B., Bourbon H., Lapeyre B., Wallace M. O., Chang J. H., Amalric F., Olson M. O. RNA binding fragments from nucleolin contain the ribonucleoprotein consensus sequence. J Biol Chem. 1987 Aug 15;262(23):10922–10925. [PubMed] [Google Scholar]
- Bugler B., Caizergues-Ferrer M., Bouche G., Bourbon H., Amalric F. Detection and localization of a class of proteins immunologically related to a 100-kDa nucleolar protein. Eur J Biochem. 1982 Nov 15;128(2-3):475–480. doi: 10.1111/j.1432-1033.1982.tb06989.x. [DOI] [PubMed] [Google Scholar]
- Caizergues-Ferrer M., Belenguer P., Lapeyre B., Amalric F., Wallace M. O., Olson M. O. Phosphorylation of nucleolin by a nucleolar type NII protein kinase. Biochemistry. 1987 Dec 1;26(24):7876–7883. doi: 10.1021/bi00398a051. [DOI] [PubMed] [Google Scholar]
- Chong M. T., Garrard W. T., Bonner J. Purification and properties of a neutral protease from rat liver chromatin. Biochemistry. 1974 Dec 3;13(25):5128–5134. doi: 10.1021/bi00722a012. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Egyhazi E., Pigon A., Chang J. H., Ghaffari S. H., Dreesen T. D., Wellman S. E., Case S. T., Olson M. O. Effects of anti-C23 (nucleolin) antibody on transcription of ribosomal DNA in Chironomus salivary gland cells. Exp Cell Res. 1988 Oct;178(2):264–272. doi: 10.1016/0014-4827(88)90397-7. [DOI] [PubMed] [Google Scholar]
- Erard M. S., Belenguer P., Caizergues-Ferrer M., Pantaloni A., Amalric F. A major nucleolar protein, nucleolin, induces chromatin decondensation by binding to histone H1. Eur J Biochem. 1988 Aug 15;175(3):525–530. doi: 10.1111/j.1432-1033.1988.tb14224.x. [DOI] [PubMed] [Google Scholar]
- Escande M. L., Gas N., Stevens B. J. Immunolocalization of the 100 K nucleolar protein in CHO cells. Biol Cell. 1985;53(2):99–109. doi: 10.1111/j.1768-322x.1985.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Gas N., Escande M. L., Stevens B. J. Immunolocalization of the 100 kDa nucleolar protein during the mitotic cycle in CHO cells. Biol Cell. 1985;53(3):209–218. doi: 10.1111/j.1768-322x.1985.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Herrera A. H., Olson M. O. Association of protein C23 with rapidly labeled nucleolar RNA. Biochemistry. 1986 Oct 7;25(20):6258–6264. doi: 10.1021/bi00368a063. [DOI] [PubMed] [Google Scholar]
- Jordan G. At the heart of the nucleolus. Nature. 1987 Oct 8;329(6139):489–490. doi: 10.1038/329489a0. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Amalric F., Ghaffari S. H., Rao S. V., Dumbar T. S., Olson M. O. Protein and cDNA sequence of a glycine-rich, dimethylarginine-containing region located near the carboxyl-terminal end of nucleolin (C23 and 100 kDa). J Biol Chem. 1986 Jul 15;261(20):9167–9173. [PubMed] [Google Scholar]
- Lapeyre B., Bourbon H., Amalric F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1472–1476. doi: 10.1073/pnas.84.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Suzuki N., Hosoya T. In vivo effects of thyroid hormone, glucocorticoid, androgen, and somatotropin on RNA polymerase I activities in isolated liver nuclei. Endocrinol Jpn. 1988 Oct;35(5):697–704. doi: 10.1507/endocrj1954.35.697. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Kirstein M. N., Wallace M. O. Limited proteolysis as a probe of the conformation and nucleic acid binding regions of nucleolin. Biochemistry. 1990 Jun 19;29(24):5682–5686. doi: 10.1021/bi00476a006. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Rivers Z. M., Thompson B. A., Kao W. Y., Case S. T. Interaction of nucleolar phosphoprotein C23 with cloned segments of rat ribosomal deoxyribonucleic acid. Biochemistry. 1983 Jul 5;22(14):3345–3351. doi: 10.1021/bi00283a007. [DOI] [PubMed] [Google Scholar]
- Pfaff M., Anderer F. A. Casein kinase II accumulation in the nucleolus and its role in nucleolar phosphorylation. Biochim Biophys Acta. 1988 Apr 2;969(1):100–109. doi: 10.1016/0167-4889(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Prestayko A. W., Klomp G. R., Schmoll D. J., Busch H. Comparison of proteins of ribosomal subunits and nucleolar preribosomal particles from Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Biochemistry. 1974 Apr 23;13(9):1945–1951. doi: 10.1021/bi00706a026. [DOI] [PubMed] [Google Scholar]
- Rao S. V., Mamrack M. D., Olson M. O. Localization of phosphorylated highly acidic regions in the NH2-terminal half of nucleolar protein C23. J Biol Chem. 1982 Dec 25;257(24):15035–15041. [PubMed] [Google Scholar]
- Schneider H. R., Issinger O. G. Growth-dependent modulation of casein kinase II and its substrate nucleolin in primary human cell cultures and HeLa cells. Biochim Biophys Acta. 1989 Oct 30;1014(1):98–100. doi: 10.1016/0167-4889(89)90246-2. [DOI] [PubMed] [Google Scholar]
- Schneider H. R., Issinger O. G. Nucleolin (C23), a physiological substrate for casein kinase II. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1390–1397. doi: 10.1016/s0006-291x(88)80786-1. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Kobayashi M., Sakata K., Suzuki T., Hosoya T. Synergistic stimulatory effect of glucocorticoid, EGF and insulin on the synthesis of ribosomal RNA and phosphorylation of nucleolin in primary cultured rat hepatocytes. Biochim Biophys Acta. 1991 May 17;1092(3):367–375. doi: 10.1016/s0167-4889(97)90014-8. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Matsui H., Hosoya T. Effects of androgen and polyamines on the phosphorylation of nucleolar proteins from rat ventral prostates with particular reference to 110-kDa phosphoprotein. J Biol Chem. 1985 Jul 5;260(13):8050–8055. [PubMed] [Google Scholar]
- Suzuki N., Saito T., Hosoya T. In vivo effects of dexamethasone and cycloheximide on the phosphorylation of 110-kDa proteins and the protein kinase activities of rat liver nucleoli. J Biol Chem. 1987 Apr 5;262(10):4696–4700. [PubMed] [Google Scholar]
- Suzuki N., Suzuki T., Uchida A., Thompson E. A., Hosoya T. Effect of dexamethasone on nucleolar casein kinase II activity and phosphorylation of nucleolin in lymphosarcoma P1798 cells. J Steroid Biochem Mol Biol. 1992 May;42(3-4):305–312. doi: 10.1016/0960-0760(92)90133-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugi K., Ogata K. Presence of a thiol protease in regenerating rat-liver nuclei. Partial purification and some properties. Eur J Biochem. 1980 Aug;109(1):9–15. doi: 10.1111/j.1432-1033.1980.tb04761.x. [DOI] [PubMed] [Google Scholar]
- Warrener P., Petryshyn R. Phosphorylation and proteolytic degradation of nucleolin from 3T3-F442A cells. Biochem Biophys Res Commun. 1991 Oct 31;180(2):716–723. doi: 10.1016/s0006-291x(05)81124-6. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Histone-H1-dependent chromatin superstructures and the suppression of gene activity. Cell. 1984 Aug;38(1):17–27. doi: 10.1016/0092-8674(84)90522-1. [DOI] [PubMed] [Google Scholar]
- Zlatanova J. Histone H1 and the regulation of transcription of eukaryotic genes. Trends Biochem Sci. 1990 Jul;15(7):273–276. doi: 10.1016/0968-0004(90)90053-e. [DOI] [PubMed] [Google Scholar]