Abstract
1. We describe the acyl-CoA and acyl-carnitine esters which arise from the incubation of well-coupled State 3 rat skeletal-muscle mitochondrial fractions with [U-14C]hexadecanoate and [U-14C]hexadecanoyl-carnitine. 2. Acyl-CoA ester intermediates of chain length 16, 14, 12, 10 and 8 carbons were detected. 3. Although incubations were in steady state in respect of oxygen consumption, 14CO2 production and generation of acid-soluble radioactivity, quantitative analysis of acyl-CoA esters showed that steady state was not achieved in respect of all intermediates. 4. 3-Hydroxyacyl- and 2-enoyl-CoA and -carnitine esters were found under normoxic conditions. 5. Direct measurement of NAD+ and NADH shows that under identical incubation conditions our observations cannot be explained by gross perturbation of the [NAD+]/[NADH] ratio. 6. We hypothesize that there is a small pool of rapidly recycling NAD+ channelled between complex I of the respiratory chain and the newly described mitochondrial-inner-membrane-associated beta-oxidation trifunctional enzyme [Uchida, Izai, Orii and Hashimoto (1992) J. Biol. Chem. 267, 1034-1041].
Full text
PDF

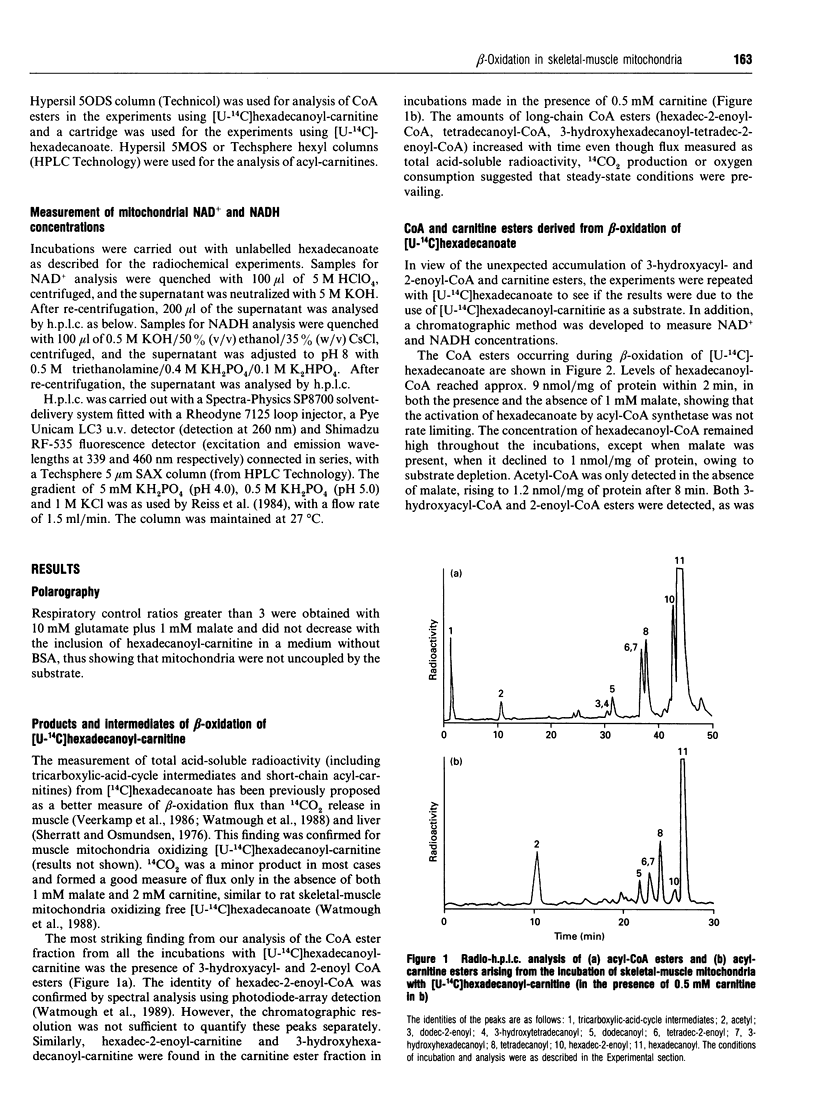
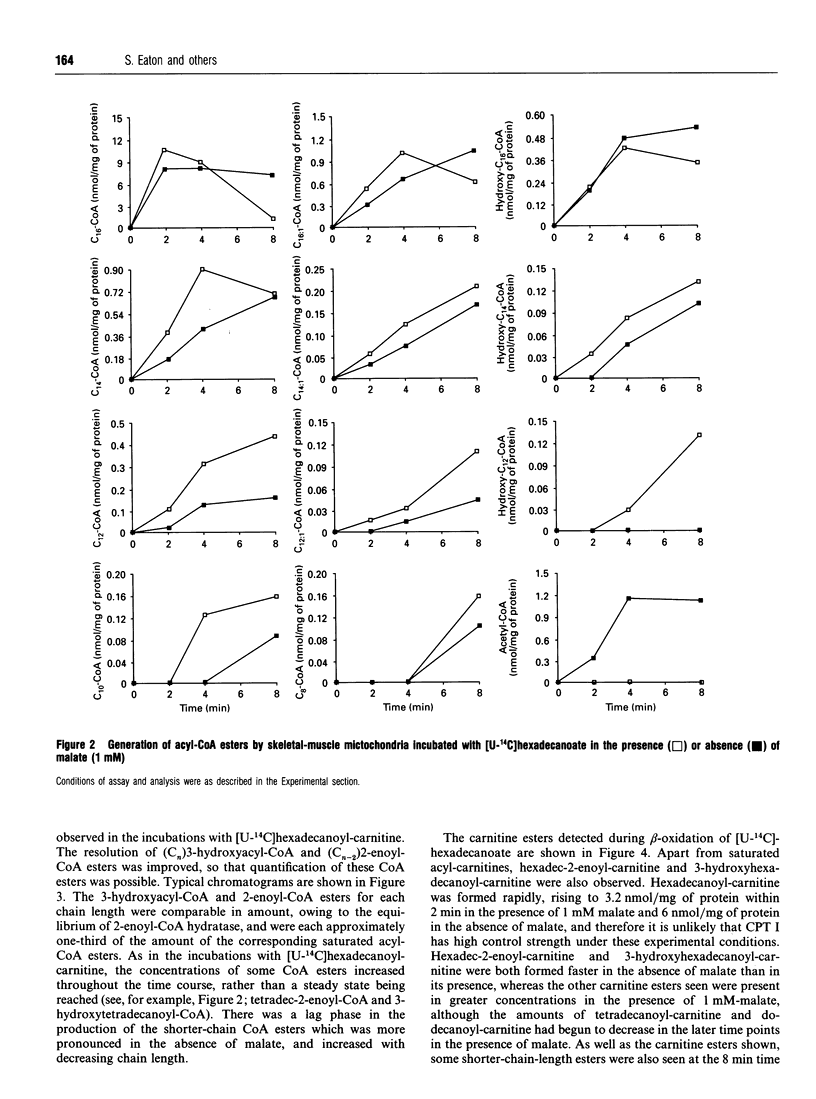
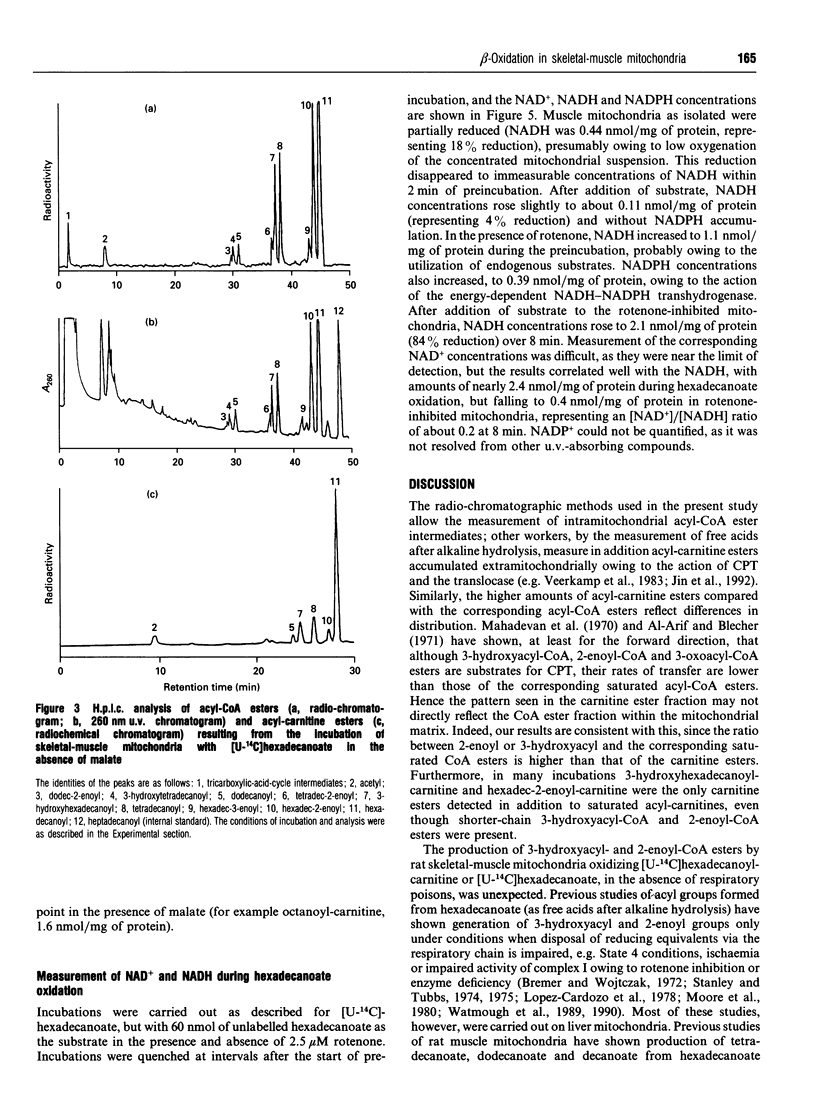
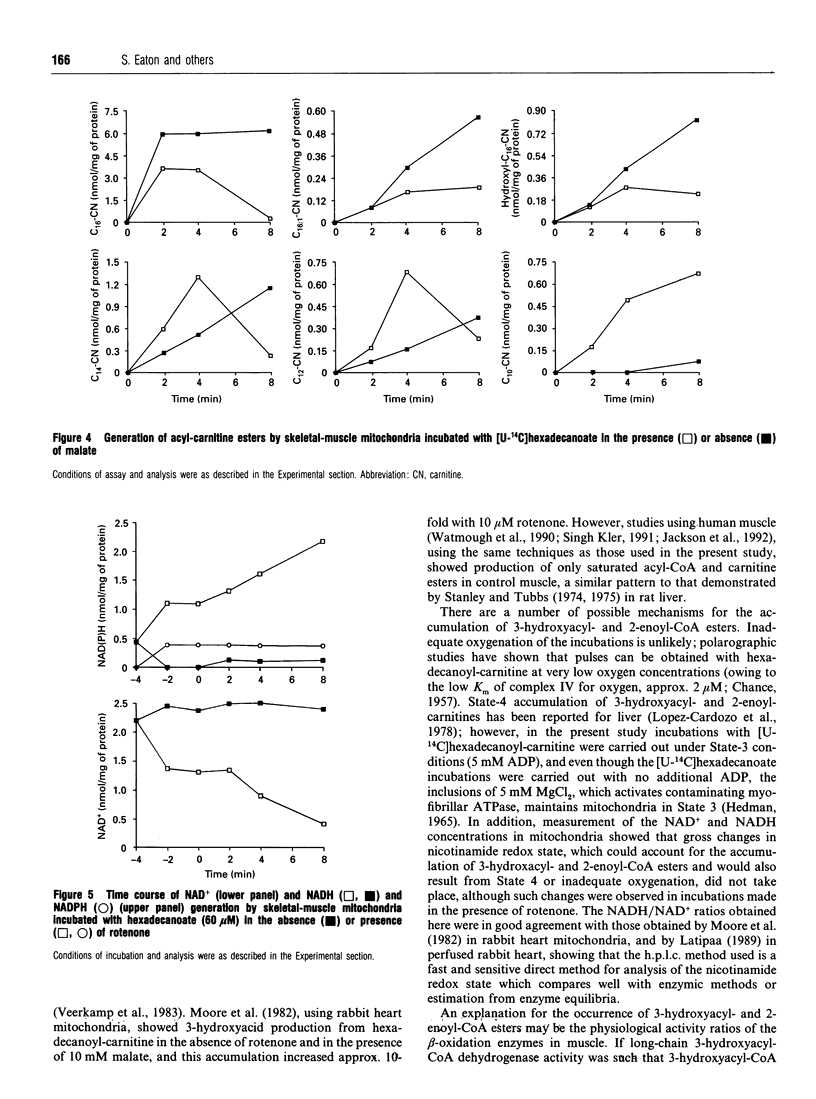


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann J. D., Frerman F. E., McKean M. C. Inhibition of general acyl CoA dehydrogenase by electron transfer flavoprotein semiquinone. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1290–1294. doi: 10.1016/s0006-291x(81)80151-9. [DOI] [PubMed] [Google Scholar]
- Bernert J. T., Jr, Sprecher H. An analysis of partial reactions in the overall chain elongation of saturated and unsaturated fatty acids by rat liver microsomes. J Biol Chem. 1977 Oct 10;252(19):6736–6744. [PubMed] [Google Scholar]
- Bhuiyan A. K., Jackson S., Turnbull D. M., Aynsley-Green A., Leonard J. V., Bartlett K. The measurement of carnitine and acyl-carnitines: application to the investigation of patients with suspected inherited disorders of mitochondrial fatty acid oxidation. Clin Chim Acta. 1992 May 15;207(3):185–204. doi: 10.1016/0009-8981(92)90118-a. [DOI] [PubMed] [Google Scholar]
- Bhuiyan A. K., Watmough N. J., Turnbull D. M., Aynsley-Green A., Leonard J. V., Bartlett K. A new simple screening method for the diagnosis of medium chain acyl-CoA dehydrogenase deficiency. Clin Chim Acta. 1987 May 29;165(1):39–44. doi: 10.1016/0009-8981(87)90216-6. [DOI] [PubMed] [Google Scholar]
- Bremer J., Wojtczak A. B. Factors controlling the rate of fatty acid -oxidation in rat liver mitochondria. Biochim Biophys Acta. 1972 Dec 8;280(4):515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- CHANCE B. Cellular oxygen requirements. Fed Proc. 1957 Sep;16(3):671–680. [PubMed] [Google Scholar]
- Carpenter K., Pollitt R. J., Middleton B. Human liver long-chain 3-hydroxyacyl-coenzyme A dehydrogenase is a multifunctional membrane-bound beta-oxidation enzyme of mitochondria. Biochem Biophys Res Commun. 1992 Mar 16;183(2):443–448. doi: 10.1016/0006-291x(92)90501-b. [DOI] [PubMed] [Google Scholar]
- Davidson B., Schulz H. Separation, properties, and regulation of acyl coenzyme A dehydrogenases from bovine heat and liver. Arch Biochem Biophys. 1982 Jan;213(1):155–162. doi: 10.1016/0003-9861(82)90450-7. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Fuel homeostasis in exercise. N Engl J Med. 1975 Nov 20;293(21):1078–1084. doi: 10.1056/NEJM197511202932107. [DOI] [PubMed] [Google Scholar]
- Fong J. C., Schulz H. Short-chain and long-chain enoyl-CoA hydratases from pig heart muscle. Methods Enzymol. 1981;71(Pt 100):390–398. doi: 10.1016/0076-6879(81)71049-8. [DOI] [PubMed] [Google Scholar]
- Frerman F. E. Reaction of electron-transfer flavoprotein ubiquinone oxidoreductase with the mitochondrial respiratory chain. Biochim Biophys Acta. 1987 Sep 10;893(2):161–169. doi: 10.1016/0005-2728(87)90035-1. [DOI] [PubMed] [Google Scholar]
- Fukushima T., Decker R. V., Anderson W. M., Spivey H. O. Substrate channeling of NADH and binding of dehydrogenases to complex I. J Biol Chem. 1989 Oct 5;264(28):16483–16488. [PubMed] [Google Scholar]
- HEDMAN R. PROPERTIES OF ISOLATED SKELETAL-MUSCLE MITOCHONDRIA FROM RAT. Exp Cell Res. 1965 Apr;38:1–12. doi: 10.1016/0014-4827(65)90422-2. [DOI] [PubMed] [Google Scholar]
- Jackson S., Bartlett K., Land J., Moxon E. R., Pollitt R. J., Leonard J. V., Turnbull D. M. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Pediatr Res. 1991 Apr;29(4 Pt 1):406–411. doi: 10.1203/00006450-199104000-00016. [DOI] [PubMed] [Google Scholar]
- Jin S. J., Hoppel C. L., Tserng K. Y. Incomplete fatty acid oxidation. The production and epimerization of 3-hydroxy fatty acids. J Biol Chem. 1992 Jan 5;267(1):119–125. [PubMed] [Google Scholar]
- Kerner J., Bieber L. Isolation of a malonyl-CoA-sensitive CPT/beta-oxidation enzyme complex from heart mitochondria. Biochemistry. 1990 May 8;29(18):4326–4334. doi: 10.1021/bi00470a010. [DOI] [PubMed] [Google Scholar]
- Kler R. S., Jackson S., Bartlett K., Bindoff L. A., Eaton S., Pourfarzam M., Frerman F. E., Goodman S. I., Watmough N. J., Turnbull D. M. Quantitation of acyl-CoA and acylcarnitine esters accumulated during abnormal mitochondrial fatty acid oxidation. J Biol Chem. 1991 Dec 5;266(34):22932–22938. [PubMed] [Google Scholar]
- Kunz W. S. Application of the theory of steady-state flux control to mitochondrial beta-oxidation. Biomed Biochim Acta. 1991;50(12):1143–1157. [PubMed] [Google Scholar]
- Kunz W. S. Evaluation of electron-transfer flavoprotein and alpha-lipoamide dehydrogenase redox states by two-channel fluorimetry and its application to the investigation of beta-oxidation. Biochim Biophys Acta. 1988 Jan 20;932(1):8–16. doi: 10.1016/0005-2728(88)90134-x. [DOI] [PubMed] [Google Scholar]
- Latipä P. M. Energy-linked regulation of mitochondrial fatty acid oxidation in the isolated perfused rat heart. J Mol Cell Cardiol. 1989 Aug;21(8):765–771. doi: 10.1016/0022-2828(89)90715-3. [DOI] [PubMed] [Google Scholar]
- Lopes-Cardozo M., Klazinga W., van den Bergh S. G. Accumulation of carnitine esters of beta-oxidation intermediates during palmitate oxidation by rat-liver mitochondria. Eur J Biochem. 1978 Feb;83(2):629–634. doi: 10.1111/j.1432-1033.1978.tb12132.x. [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Malaiyandi M., Erfle J. D., Sauer F. Metabolism of L-carnitine esters of beta-substituted palmitic acid by rat liver mitochondria. J Biol Chem. 1970 Jun;245(12):3218–3224. [PubMed] [Google Scholar]
- Moore K. H., Radloff J. F., Hull F. E., Sweeley C. C. Incomplete fatty acid oxidation by ischemic heart: beta-hydroxy fatty acid production. Am J Physiol. 1980 Aug;239(2):H257–H265. doi: 10.1152/ajpheart.1980.239.2.H257. [DOI] [PubMed] [Google Scholar]
- Moore K. H., Radloff J. F., Koen A. E., Hull F. E. Incomplete fatty acid oxidation by heart mitochondria: beta-hydroxy fatty acid production. J Mol Cell Cardiol. 1982 Aug;14(8):451–459. doi: 10.1016/0022-2828(82)90151-1. [DOI] [PubMed] [Google Scholar]
- Oram J. F., Bennetch S. L., Neely J. R. Regulation of fatty acid utilization in isolated perfused rat hearts. J Biol Chem. 1973 Aug 10;248(15):5299–5309. [PubMed] [Google Scholar]
- Pande S. V. On rate-controlling factors of long chain fatty acid oxidation. J Biol Chem. 1971 Sep 10;246(17):5384–5390. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pourfarzam M., Bartlett K. Products and intermediates of the beta-oxidation of [U-14C]hexadecanedionoyl-mono-CoA by rat liver peroxisomes and mitochondria. Biochem J. 1991 Jan 1;273(Pt 1):205–210. doi: 10.1042/bj2730205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell P. J., Lau S. M., Killian D., Thorpe C. Interaction of acyl coenzyme A substrates and analogues with pig kidney medium-chain acyl-coA dehydrogenase. Biochemistry. 1987 Jun 16;26(12):3704–3710. doi: 10.1021/bi00386a066. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Derrick J. P., Friend A. S., Tubbs P. K. Purification and properties of the soluble carnitine palmitoyltransferase from bovine liver mitochondria. Biochem J. 1987 Jun 1;244(2):271–278. doi: 10.1042/bj2440271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann H., De Vivo D. C. Coordinate enzymatic activity of beta-oxidation and purine nucleotide cycle in a diversity of muscle and other organs of rat. Comp Biochem Physiol B. 1991;98(2-3):327–331. doi: 10.1016/0305-0491(91)90186-h. [DOI] [PubMed] [Google Scholar]
- Reiss P. D., Zuurendonk P. F., Veech R. L. Measurement of tissue purine, pyrimidine, and other nucleotides by radial compression high-performance liquid chromatography. Anal Biochem. 1984 Jul;140(1):162–171. doi: 10.1016/0003-2697(84)90148-9. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981 Jul 6;129(2):229–232. doi: 10.1016/0014-5793(81)80171-8. [DOI] [PubMed] [Google Scholar]
- Sherratt H. S., Watmough N. J., Johnson M. A., Turnbull D. M. Methods for study of normal and abnormal skeletal muscle mitochondria. Methods Biochem Anal. 1988;33:243–335. doi: 10.1002/9780470110546.ch6. [DOI] [PubMed] [Google Scholar]
- Stanley H., Sherratt A., Osmundsen H. On the mechanisms of some pharmacological actions of the hypoglycaemic toxins hypoglycin and pent-4-enoic acid. A way out of the present confusion. Biochem Pharmacol. 1976 Apr 1;25(7):743–750. doi: 10.1016/0006-2952(76)90139-8. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Tubbs P. K. The occurrence of intermediates in mitochondrial fatty acid oxidation. FEBS Lett. 1974 Mar 1;39(3):325–328. doi: 10.1016/0014-5793(74)80141-9. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Tubbs P. K. The role of intermediates in mitochondrial fatty acid oxidation. Biochem J. 1975 Jul;150(1):77–88. doi: 10.1042/bj1500077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumegi B., Srere P. A. Complex I binds several mitochondrial NAD-coupled dehydrogenases. J Biol Chem. 1984 Dec 25;259(24):15040–15045. [PubMed] [Google Scholar]
- Uchida Y., Izai K., Orii T., Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J Biol Chem. 1992 Jan 15;267(2):1034–1041. [PubMed] [Google Scholar]
- Veerkamp J. H., Van Moerkerk H. T., Glatz J. F., Van Hinsbergh V. W. Incomplete palmitate oxidation in cell-free systems of rat and human muscles. Biochim Biophys Acta. 1983 Oct 11;753(3):399–410. doi: 10.1016/0005-2760(83)90064-4. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H., van Moerkerk T. B., Glatz J. F., Zuurveld J. G., Jacobs A. E., Wagenmakers A. J. 14CO2 production is no adequate measure of [14C]fatty acid oxidation. Biochem Med Metab Biol. 1986 Jun;35(3):248–259. doi: 10.1016/0885-4505(86)90080-0. [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Baxter C. F., Jr, Schulz H. Regulation of fatty acid beta-oxidation in rat heart mitochondria. Arch Biochem Biophys. 1991 Sep;289(2):274–280. doi: 10.1016/0003-9861(91)90472-u. [DOI] [PubMed] [Google Scholar]
- Waterson R. M., Hill R. L. Enoyl coenzyme A hydratase (crotonase). Catalytic properties of crotonase and its possible regulatory role in fatty acid oxidation. J Biol Chem. 1972 Aug 25;247(16):5258–5265. [PubMed] [Google Scholar]
- Watmough N. J., Bhuiyan A. K., Bartlett K., Sherratt H. S., Turnbull D. M. Skeletal muscle mitochondrial beta-oxidation. A study of the products of oxidation of [U-14C]hexadecanoate by h.p.l.c. using continuous on-line radiochemical detection. Biochem J. 1988 Jul 15;253(2):541–547. doi: 10.1042/bj2530541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watmough N. J., Bindoff L. A., Birch-Machin M. A., Jackson S., Bartlett K., Ragan C. I., Poulton J., Gardiner R. M., Sherratt H. S., Turnbull D. M. Impaired mitochondrial beta-oxidation in a patient with an abnormality of the respiratory chain. Studies in skeletal muscle mitochondria. J Clin Invest. 1990 Jan;85(1):177–184. doi: 10.1172/JCI114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watmough N. J., Turnbull D. M., Sherratt H. S., Bartlett K. Measurement of the acyl-CoA intermediates of beta-oxidation by h.p.l.c. with on-line radiochemical and photodiode-array detection. Application to the study of [U-14C]hexadecanoate oxidation by intact rat liver mitochondria. Biochem J. 1989 Aug 15;262(1):261–269. doi: 10.1042/bj2620261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Y., He X. Y., Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem. 1987 Sep 25;262(27):13027–13032. [PubMed] [Google Scholar]


