Abstract
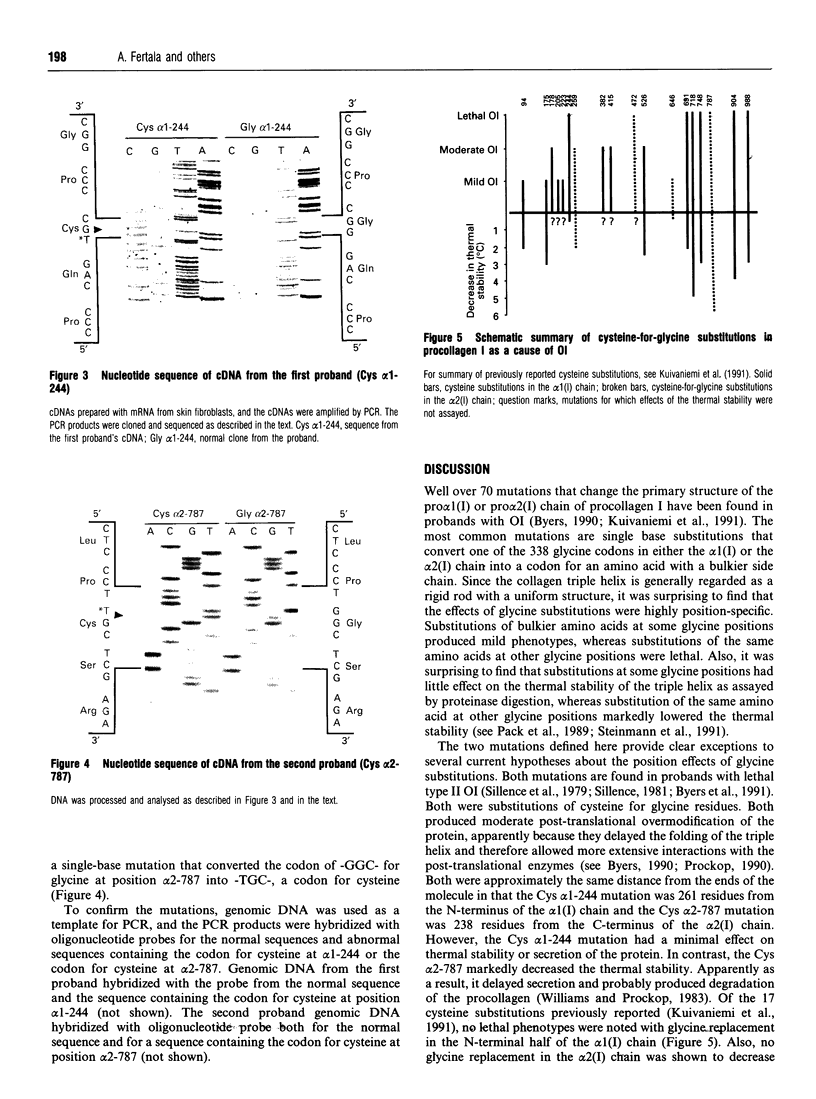
Cultured skin fibroblasts were examined from two probands with type II (lethal) osteogenesis imperfecta. One proband had a single base mutation which converted the glycine codon at position alpha 1-244 in the alpha 1(I) chain of procollagen I into a cysteine codon whereas the other had a similar mutation that converted the glycine codon at position alpha 2-787 of the alpha 2(I) chain into a cysteine codon. Both mutations produced post-translational overmodification of procollagen I. The Cys alpha 1-244 mutation, however, had a minimal effect on the thermal stability or secretion of the protein whereas the Cys alpha 2-787 mutation markedly decreased the thermal stability and, apparently as a result, essentially none of the mutated protein was secreted. The results provide clear exceptions to two previous generalizations about the position-specificity of glycine substitutions in procollagen I.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Byers P. H. Brittle bones--fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990 Sep;6(9):293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Wallis G. A., Willing M. C. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet. 1991 Jul;28(7):433–442. doi: 10.1136/jmg.28.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Pack M., Constantinou C. D., Kalia K., Nielsen K. B., Prockop D. J. Substitution of serine for alpha 1(I)-glycine 844 in a severe variant of osteogenesis imperfecta minimally destabilizes the triple helix of type I procollagen. The effects of glycine substitutions on thermal stability are either position of amino acid specific. J Biol Chem. 1989 Nov 25;264(33):19694–19699. [PubMed] [Google Scholar]
- Prockop D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990 Sep 15;265(26):15349–15352. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D. Osteogenesis imperfecta: an expanding panorama of variants. Clin Orthop Relat Res. 1981 Sep;(159):11–25. [PubMed] [Google Scholar]
- Steinmann B., Rao V. H., Vogel A., Bruckner P., Gitzelmann R., Byers P. H. Cysteine in the triple-helical domain of one allelic product of the alpha 1(I) gene of type I collagen produces a lethal form of osteogenesis imperfecta. J Biol Chem. 1984 Sep 10;259(17):11129–11138. [PubMed] [Google Scholar]
- Steinmann B., Westerhausen A., Constantinou C. D., Superti-Furga A., Prockop D. J. Substitution of cysteine for glycine-alpha 1-691 in the pro alpha 1(I) chain of type I procollagen in a proband with lethal osteogenesis imperfecta destabilizes the triple helix at a site C-terminal to the substitution. Biochem J. 1991 Nov 1;279(Pt 3):747–752. doi: 10.1042/bj2790747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studencki A. B., Wallace R. B. Allele-specific hybridization using oligonucleotide probes of very high specific activity: discrimination of the human beta A- and beta S-globin genes. DNA. 1984;3(1):7–15. doi: 10.1089/dna.1.1984.3.7. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Steinmann B. Impaired secretion of type III procollagen in Ehlers-Danlos syndrome type IV fibroblasts: correction of the defect by incubation at reduced temperature and demonstration of subtle alterations in the triple-helical region of the molecule. Biochem Biophys Res Commun. 1988 Jan 15;150(1):140–147. doi: 10.1016/0006-291x(88)90497-4. [DOI] [PubMed] [Google Scholar]
- Torre-Blanco A., Adachi E., Romanic A. M., Prockop D. J. Copolymerization of normal type I collagen with three mutated type I collagens containing substitutions of cysteine at different glycine positions in the alpha 1 (I) chain. J Biol Chem. 1992 Mar 5;267(7):4968–4973. [PubMed] [Google Scholar]
- Vogel B. E., Doelz R., Kadler K. E., Hojima Y., Engel J., Prockop D. J. A substitution of cysteine for glycine 748 of the alpha 1 chain produces a kink at this site in the procollagen I molecule and an altered N-proteinase cleavage site over 225 nm away. J Biol Chem. 1988 Dec 15;263(35):19249–19255. [PubMed] [Google Scholar]
- Westerhausen A. I., Constantinou C. D., Prockop D. J. A sequence polymorphism in the 3'-nontranslated region of the pro alpha 1 chain of type I procollagen. Nucleic Acids Res. 1990 Aug 25;18(16):4968–4968. doi: 10.1093/nar/18.16.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet W. J., Pihlajaniemi T., Myers J., Kelly T. E., Prockop D. J. Synthesis of a shortened pro-alpha 2(I) chain and decreased synthesis of pro-alpha 2(I) chains in a proband with osteogenesis imperfecta. J Biol Chem. 1983 Jun 25;258(12):7721–7728. [PubMed] [Google Scholar]