Abstract
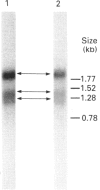
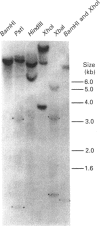
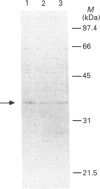
Dehydroepiandrosterone sulphotransferase (DHEA-ST) catalyses the 3'-phosphoadenosine 5'-phosphosulphate-dependent sulphation of a wide variety of steroids in human liver and adrenal tissue and is responsible for most, if not all, of the sulphation of bile acids in human liver. This report describes the isolation, characterization and expression of a cDNA which encodes human liver DHEA-ST. The DHEA-ST cDNA, designated DHEA-ST8, was isolated from a Uni-Zap XR human liver cDNA library and is composed of 1060 bp and contains an open reading frame encoding a 285-amino-acid protein with a molecular mass of approx. 33765 Da. Translation of DHEA-ST8 in vitro generated a protein identical in molecular size with that of DHEA-ST. Expression of DHEA-ST8 in COS-7 cells produces an active DHEA-ST protein which is capable of sulphating DHEA, has the same molecular mass as human liver DHEA-ST and is recognized by rabbit anti-(human liver DHEA-ST) antibodies. Northern-blot analysis of human liver RNA detects the presence of three different size transcripts; however, Southern-blot analysis of human DNA suggests that only one gene may be present in the genome. These results describe the cloning of a human ST which has an important role in the sulphation of steroids and bile acids in human liver and adrenals.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. B. Enzymic synthesis of steroid sulphates. XVII. On the structure of bovine estrogen sulphotransferase. Biochim Biophys Acta. 1991 Jan 29;1076(2):282–288. doi: 10.1016/0167-4838(91)90279-9. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E., Khaw K. T., Yen S. S. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986 Dec 11;315(24):1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- Chatterjee B., Majumdar D., Ozbilen O., Murty C. V., Roy A. K. Molecular cloning and characterization of cDNA for androgen-repressible rat liver protein, SMP-2. J Biol Chem. 1987 Jan 15;262(2):822–825. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins R. H., Lack L., Harman K. M., Killenberg P. G. Rat hepatic bile acid sulfotransferase: identification of the catalytic polypeptide and evidence for polymeric forms in female rats. Hepatology. 1986 Jul-Aug;6(4):579–586. doi: 10.1002/hep.1840060406. [DOI] [PubMed] [Google Scholar]
- Comer K. A., Falany C. N. Immunological characterization of dehydroepiandrosterone sulfotransferase from human liver and adrenal. Mol Pharmacol. 1992 Apr;41(4):645–651. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falany C. N., Vazquez M. E., Kalb J. M. Purification and characterization of human liver dehydroepiandrosterone sulphotransferase. Biochem J. 1989 Jun 15;260(3):641–646. doi: 10.1042/bj2600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshey S. J., Dooley T. P., Reardon I. M., Heinrikson R. L., Falany C. N. Sequence analysis, in vitro translation, and expression of the cDNA for rat liver minoxidil sulfotransferase. Mol Pharmacol. 1992 Aug;42(2):257–264. [PubMed] [Google Scholar]
- Hobkirk R., Glasier M. A., Brown L. Y. Purification and some characteristics of an oestrogen sulphotransferase from guinea pig adrenal gland and its non-identity with adrenal pregnenolone sulphotransferase. Biochem J. 1990 Jun 15;268(3):759–764. doi: 10.1042/bj2680759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Sano H., Ikeuchi T., Sakyo K., Hirakawa S., Mori Y. Effect of dehydroepiandrosterone sulfate on collagenase production in rabbit uterine cervix culture. Biochem Med. 1984 Apr;31(2):257–266. doi: 10.1016/0006-2944(84)90031-0. [DOI] [PubMed] [Google Scholar]
- Kane R. E., Chen L. J., Herbst J. J., Thaler M. M. Sexual differentiation of rat hepatic bile salt sulfotransferase isoenzymes. Pediatr Res. 1988 Aug;24(2):247–253. doi: 10.1203/00006450-198808000-00022. [DOI] [PubMed] [Google Scholar]
- Lyon E. S., Marcus C. J., Wang J. L., Jakoby W. B. Hydroxysteroid sulfotransferase. Methods Enzymol. 1981;77:206–213. doi: 10.1016/s0076-6879(81)77027-7. [DOI] [PubMed] [Google Scholar]
- Moore S. S., Thompson E. O., Nash A. R. Oestrogen sulfotransferase: isolation of a high specific activity species from bovine placenta. Aust J Biol Sci. 1988;41(3):333–341. doi: 10.1071/bi9880333. [DOI] [PubMed] [Google Scholar]
- Nash A. R., Glenn W. K., Moore S. S., Kerr J., Thompson A. R., Thompson E. O. Oestrogen sulfotransferase: molecular cloning and sequencing of cDNA for the bovine placental enzyme. Aust J Biol Sci. 1988;41(4):507–516. doi: 10.1071/bi9880507. [DOI] [PubMed] [Google Scholar]
- Nestler J. E., Barlascini C. O., Clore J. N., Blackard W. G. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab. 1988 Jan;66(1):57–61. doi: 10.1210/jcem-66-1-57. [DOI] [PubMed] [Google Scholar]
- Ogura K., Kajita J., Narihata H., Watabe T., Ozawa S., Nagata K., Yamazoe Y., Kato R. Cloning and sequence analysis of a rat liver cDNA encoding hydroxysteroid sulfotransferase. Biochem Biophys Res Commun. 1989 Nov 30;165(1):168–174. doi: 10.1016/0006-291x(89)91050-4. [DOI] [PubMed] [Google Scholar]
- Ogura K., Kajita J., Narihata H., Watabe T., Ozawa S., Nagata K., Yamazoe Y., Kato R. cDNA cloning of the hydroxysteroid sulfotransferase STa sharing a strong homology in amino acid sequence with the senescence marker protein SMP-2 in rat livers. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1494–1500. doi: 10.1016/0006-291x(90)91036-r. [DOI] [PubMed] [Google Scholar]
- Ogura K., Sohtome T., Sugiyama A., Okuda H., Hiratsuka A., Watabe T. Rat liver cytosolic hydroxysteroid sulfotransferase (sulfotransferase a) catalyzing the formation of reactive sulfate esters from carcinogenic polycyclic hydroxymethylarenes. Mol Pharmacol. 1990 Jun;37(6):848–854. [PubMed] [Google Scholar]
- Radominska A., Comer K. A., Zimniak P., Falany J., Iscan M., Falany C. N. Human liver steroid sulphotransferase sulphates bile acids. Biochem J. 1990 Dec 15;272(3):597–604. doi: 10.1042/bj2720597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Nakano R., Kadoya Y., Iwao M., Shima K., Sowa M. Cervical ripening with dehydroepiandrosterone sulphate. Br J Obstet Gynaecol. 1982 Mar;89(3):195–198. doi: 10.1111/j.1471-0528.1982.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Kasper C. B. Genetic polymorphisms for a phenobarbital-inducible cytochrome P-450 map to the Coh locus in mice. J Biol Chem. 1983 Aug 25;258(16):9585–9588. [PubMed] [Google Scholar]
- Singer S. S., Giera D., Johnson J., Sylvester S. Enzymatic sulfation of steroids: I. The enzymatic basis for the sex difference in cortisol sulfation by rat liver preparations. Endocrinology. 1976 Apr;98(4):963–974. doi: 10.1210/endo-98-4-963. [DOI] [PubMed] [Google Scholar]
- Yuen S. W., Chui A. H., Wilson K. J., Yuan P. M. Microanalysis of SDS-PAGE electroblotted proteins. Biotechniques. 1989 Jan;7(1):74–83. [PubMed] [Google Scholar]