Abstract
OBJECTIVES:
Critically ill adults requiring artificial airways experience profound communication deficits. Studies of interventions supporting communication report disparate outcomes, creating subsequent challenges in the interpretation of their effectiveness. Therefore, we aimed to develop international consensus for a communication core outcome set (Comm-COS) for future trials of communication interventions in this population.
DESIGN:
1) Systematic review, 2) patient/family interviews, 3) two-round modified Delphi, and 4) virtual consensus meetings with a final voting round. A multidisciplinary expert steering committee oversaw all stages.
SETTING:
Interviews and consensus meetings were conducted via videoconferencing. Digital methods were used for Delphi and final Comm-COS voting.
SUBJECTS:
Three stakeholder groups: 1) patient and family members with lived experience within 3 years, 2) clinicians with experience working in critical care, and 3) researchers publishing in the field.
INTERVENTION:
None.
MEASUREMENTS AND MAIN RESULTS:
We identified 59 outcomes via our systematic review, 3 unique outcomes from qualitative interviews, and 2 outcomes from our steering committee. Following item reduction, 32 outcomes were presented in Delphi round 1; 134 participants voted; 15 patient/family (11%), 91 clinicians (68%), and 28 researchers (21%). Nine additional outcomes were generated and added to round 2; 106 (81%) participants voted. Following completion of the consensus processes, the Comm-COS includes seven outcomes: 1) changes in emotions and wellbeing associated with ability to communicate, 2) physical impact of communication aid use, 3) time to functional communication, 4) ability to communicate healthcare needs (comfort/care/safety/decisions), 5) conversation agency, 6) ability to establish a communication connection to develop and maintain relationships, and 7) acceptability of the communication intervention.
CONCLUSIONS:
This is the first COS to specifically focus on communication for critically ill adults. Limitations for operationalization include selection of measures to use with these outcomes. Identification of suitable measures and adoption of the Comm-COS in future trials will help establish effective interventions to ameliorate the highly prevalent and negative experience of communicative incapacity.
Keywords: airway management, clinical trials, communication, core outcome set, critical care
KEY POINTS.
Question: What outcomes should be included in a core outcome set for future trials enabling communication for critically ill adults with an artificial airway, with or without mechanical ventilation?
Findings: Following a rigorous multiple stakeholder study process including a systematic review, qualitative interviews, two-round Delphi, and consensus meetings; seven outcomes were included in the final communication core outcome set (Comm-COS).
Meaning: The Comm-COS provides a minimum outcome set for researchers to use in all future communication trials, which will increase the consistency of the evidence base enabling better understanding of effective interventions.
For critically ill patients requiring an artificial airway (i.e., endotracheal or tracheostomy tube), establishing and maintaining communication is particularly challenging (1, 2). Effective communication in this population has recently been defined, via an international multiprofessional consensus, as the degree in which a patient can initiate, impart, receive, and understand information, and can range from an ineffective to effective exchange of basic to complex information between the patient and the communication partner(s) (3). Barriers to effective communication are multifactorial and include: loss of voice due to the artificial airway, muscular weakness, acute cognitive changes, the ICU environment, and lack of access to effective communication interventions to meet diverse communication needs (1, 4–7). These challenges to communication are key stressors for patients (2, 6, 8). Ineffective communication has negative outcomes including emotional distress, unrecognized pain and delirium, misinterpretation of messaging, and reduced ability to be involved in healthcare decisions (2, 6, 9–14). Furthermore, negative outcomes persist beyond the ICU admission itself, with patients reporting persistent communicative deficits including ongoing changes to voice and cognitive deficits affecting communicative abilities following hospital discharge (15).
For patients with artificial airways, a variety of interventions (e.g., one-way speaking valve) and alternative and augmentative communication (AAC) exist, with new options emerging with advancing technology and artificial intelligence (e.g., SRAVI.ai–artificial intelligence for lip reading). Despite the range of low-tech AAC (e.g., writing equipment, communication boards) to high-tech AAC (e.g., speech generating devices, using direct access, switches, or eye-gaze) that might be made available, the evidence for effective interventions to enable communication for critically ill patients with an artificial airway is limited (16). Heterogeneity across studies of selected outcomes and use of measures with untested psychometric properties contributes to difficulty in synthesizing the evidence to date to provide guidance for clinicians (16, 17).
Core outcome sets (COS) aim to standardize outcome reporting by identifying outcomes perceived to be fundamental for measurement in trials of a specific interest area. Study outcomes are processes or events hypothesized to be modified by an intervention and are used in trials to compare the effects of different interventions (18). Outcomes included in a COS should be relevant and important to patients, clinicians, key decision-makers, and researchers maximizing the uptake and use of COS across studies and increasing comparability of findings (18, 19). Although there is increasing acceptance of the importance and value of COS within critical care, to date there are only a small number developed (20), and none specifically focusing on communication. The aim of this research was to establish a Communication COS (Comm-COS) for studies of interventions designed to enable communication in critically ill adults requiring an artificial airway with or without mechanical ventilatory support.
MATERIALS AND METHODS
The Comm-COS was developed in four stages: 1) systematic review of outcomes published in peer reviewed literature, 2) semi-structured interviews with patients and family members, 3) two-round online modified Delphi with international representation, and 4) a series of four virtual consensus meetings with a final round of anonymous voting. The Comm-COS was developed and registered with the Core Outcome Measures in Effectiveness Trials (COMET) Initiative (http://www.comet-initiative.org/Studies/Details/1671), and endorsed by the U.K. Intensive Care Society (ICS), the European Society of Intensive Care Medicine (ESICM), and ICUsteps. All stages of the Comm-COS development were conducted according to the COMET Handbook (21) and are reported following the Core Outcome Set-STAndards for Reporting (22). All aspects of the Comm-COS study were reviewed and approved by the University of Technology Sydney Ethics Review Committee on May 26, 2021 (ID: ETH21-5966; Study name: Development of a Core Outcome Measurement Set for Studies of Interventions to Enable Communication in Adults Requiring an Artificial Airway With or Without Mechanical Ventilatory support). All study procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975.
PARTICIPANTS
Steering Committee
We convened an international expert steering committee (ESC) with invitations sent to ten identified content experts. We invited experts with a record of critical care clinical experience and publications in the peer reviewed literature on enabling communication in critically ill patients (clinician researchers), or lived experience communicating with an artificial airway (patients/family members). The 10 members of the international multidisciplinary steering committee were clinician researchers (n = 9), and patients/family members (n = 1). Clinician researchers represented nursing, medicine, physiotherapy, and speech language pathology professions from Australia, Canada, The Netherlands, United Kingdom, and United States of America. The Comm-COS steering committee endorsed the study protocol and were consulted throughout the Comm-COS development.
Participants for Semi-Structured Interviews, Delphi and Consensus Meetings
Using purposive sampling, we recruited participants to three stakeholder groups: 1) patients and family members, 2) clinicians, and 3) clinician researchers. We used multiple recruitment strategies including social media via Twitter, Intensive Care Professional Society email/newsletters and webpages (e.g., ICS and ESICM), patient advocacy groups (e.g., ICUsteps in the United Kingdom), clinical networks (e.g., Trache Clinical Education Network), emails to personal and professional contacts, and snowballing methods (23).
Inclusion criteria were: “patients and family members”: 1) adults older than 18 years who had an artificial airway in the previous 3 years, 2) family members or friends (communication partners) of an individual who required an artificial airway, 3) members of a patient user/support group. “Clinicians” 1) eligible for membership/registration of their designated professional society, 2) at least 2 years clinical experience working with people with an artificial airway. “Researchers” 1) national standing or published research relevant to the field. For pragmatic reasons, as the research was conducted in English, all participants were required to understand, read, and speak English. There were no exclusion criteria.
Evidence Review
We conducted a systematic review of outcomes reported in published trials to commence the Comm-COS item generation process. This systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (24) and was registered prospectively on Open Science Framework (https://osf.io/pkzy9). In brief, our search strategy combined the search strategies of two recently published reviews on communication aids for critically ill patients with artificial airways (7, 17). We then searched five databases to identify studies published from March 2019 to September 2021. Two authors (A.F.S., R.S.) independently screened citations, and full text papers (A.F.S., R.S.), with a third resolving any disagreements (L.R.). Five researchers (in pairs) (A.F.S., A.L.S., L.I., R.S., A.M.) independently extracted data using a purpose designed data extraction tool developed by the review team, all extractions were additional verified by one author (R.S.). All studies in the two reviews (7, 17) were also reviewed for inclusion and selected if they reported outcomes and measures used to evaluate a communication intervention.
Patient and Family Interviews
An experienced researcher (A.F.S.) conducted interviews via videoconference (Zoom) using a semi-structured interview guide with questions focusing on the identification of outcomes, the experience of communicating with an artificial airway, most important communication messages, and the communicative context including communication partners. Interviews were digitally recorded, transcribed verbatim, and analyzed using content analysis (25), with verification by a second experienced researcher (L.R.), to identify outcomes for inclusion in the first Delphi round.
Modified Delphi
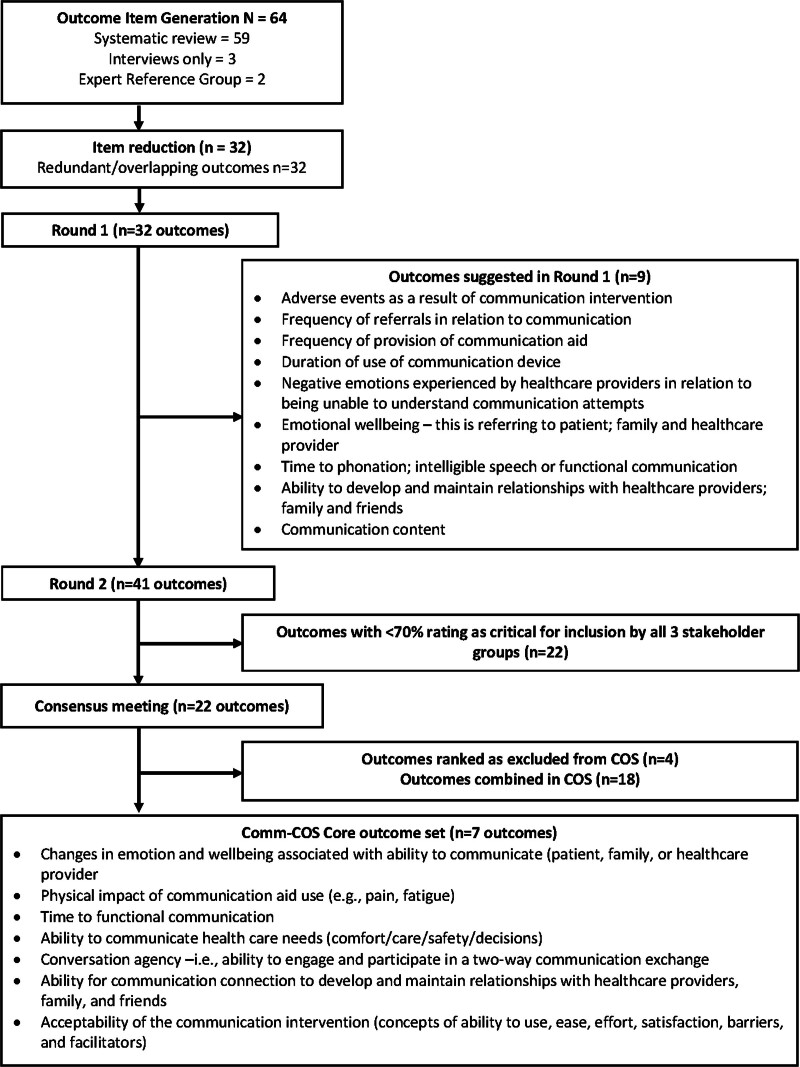
To inform the Delphi round 1, we removed duplicate outcomes and combined those for which we perceived some overlapping of concepts (e.g., depression and frustration were combined as the outcome “negative emotions”) (Fig. 1). We further refined the outcome list and lay descriptions following feedback from the steering committee. We then categorized the outcome list using the COMET Outcome Classification Taxonomy domains to generate the Delphi round 1 questionnaire (26). Outcome domains were presented in random order to minimize selection bias.
Figure 1.
Outcome selection processes. COS = core outcome set.
We followed a two round Delphi process using the bespoke DelphiManager (V5.0 software; COMET initiative, Liverpool, United Kingdom). Participants self-identified their preferred stakeholder group, within the inclusion criteria for patient/family member, clinician, or researcher; providing demographic data on Delphi commencement. Participants were asked to rate the importance of each outcome using the Grading of Recommendations Assessment, Development and Evaluation Scale. In this 9-point ordinal scale, a score of 1–3 is considered not important, a scores 4–6 important but not critical, and a score of 7–9 important and critical. Participants were offered an option of scoring an item not applicable, which did not carry an importance score. Participants could also suggest additional outcomes for consideration in subsequent rounds.
On round 1 completion, the calculations were made of the proportion of participants scoring each outcome in the three importance categories as well as the median (interquartile range) scores overall and for each of the three stakeholder groups. Histograms were created to display the data visually for round 2. We considered all additionally suggested outcomes against the definition of an outcome, and decisions on inclusion/exclusion were reviewed by the steering committee.
In round 2, participants were presented with their round 1 rating of each outcome and a histogram displaying the ratings of each stakeholder group. Participants were asked to repeat the rating process, considering their prior scores and the feedback on scoring from the stakeholder groups. Participants were requested to provide a rationale if their rating of importance had moved from one importance category to another compared with the prior round. All outcomes suggested and added to round 2 were rated on two occasions by participants in line with aforementioned methods. Each Delphi round was open for 8 weeks, with participants receiving a maximum of three reminders before round closure.
Consensus Meetings
We held a series of four consensus meetings, timed to accommodate various time zones, with all voting participants in the Delphi invited to attend one meeting option. Each meeting was moderated by a minimum of two steering group members, digitally recorded, and transcribed. Using a modified nominal group technique (27), we first presented outcomes scored between 7 and 9 by greater than or equal to 70% and between 1 and 3 by less than 15% of participants across all stakeholder groups. We then held rounds of iterative discussions to rank outcomes using Google Jamboard (Google) as a visual facilitator. In subsequent meetings after iterative group discussion, the results of prior consensus meetings were presented, to enable a collaborative consensus discussion approach across meetings. Lastly, the meeting results were summarized into a Word document which was distributed via email with a final request to vote using the Research Electronic Data Capture (Vanderbilt University, Nashville, TN) survey (28) on the selected outcomes for inclusion in the COS. A priori, we selected a criterion of greater than or equal to 50% of participants to vote yes for an outcome to be included in the Comm-COS (29, 30).
RESULTS
Item Generation Phase
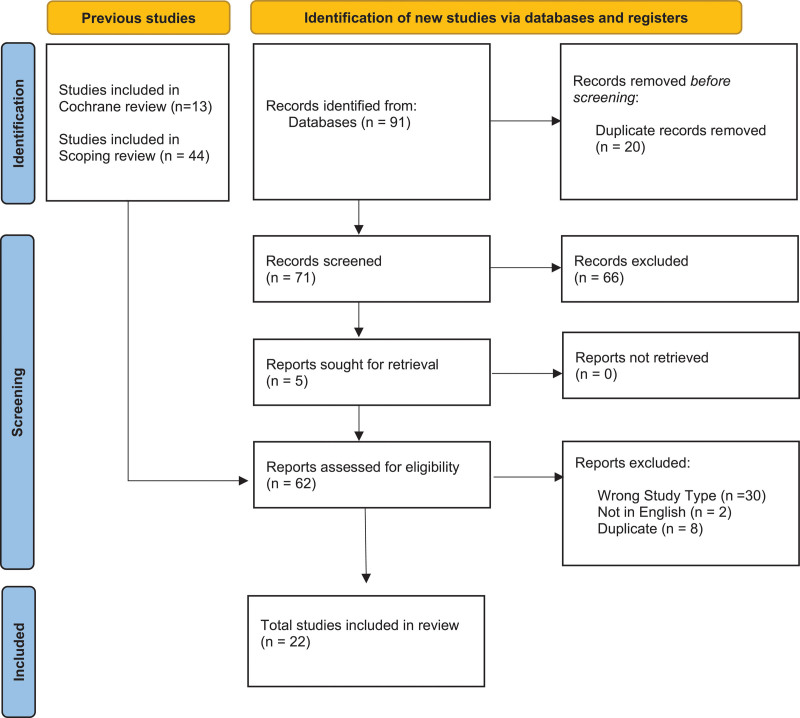
Our systematic review identified 22 papers meeting our inclusion criteria (Fig. 2). From these, 59 outcomes were identified (Table E1, http://links.lww.com/CCM/H556). In total, there were 14 outcomes identified from interviews (average length of interview 51 min) with 15 former patients or family members (Table 1), with three of these outcomes being unique from those identified in the systematic review. Following review and iterative discussions with the project’s steering group, we included 32 outcomes across 11 COMET taxonomy categories in the Delphi round 1 (Table E2, http://links.lww.com/CCM/H556).
Figure 2.
Preferred Reporting Items for Systematic reviews and Meta-Analyses flowchart.
TABLE 1.
Outcomes Identified by Patients and Families With Lived Experience
| Outcome (N = 15 Participants) | n (%) |
|---|---|
| Communication ease/effort | 15 (100) |
| Negative emotions associated with inability to communicate | 15 (100) |
| Ability to communicate in a manner that means my intentions and needs are understood by my communication partner(s) | 14 (93) |
| Ability to participate in a conversation on my chosen topic | 14 (93) |
| Ability to communicate my care, comfort, and safety needs | 12 (80) |
| Positive emotions due to ability to communicatea | 12 (80) |
| Ability to communicate at a normal speeda | 9 (60) |
| Satisfaction with communication intervention | 7 (47) |
| Ability to participate in care decision-makinga | 6 (40) |
| Ability to gain attention (of my care team/family member)a | 6 (40) |
| Ability to generate audible voice (intervention specific) | 5 (33) |
| Ability of the patient to communicate when they want to, that is, availability of device | 4 (27) |
| Fatigue related to a communication intervention | 4 (27) |
| Pain or discomfort related to use of communication intervention | 1 (6) |
Not identified in the systematic review.
Consensus Building Phase
We recruited 134 Delphi participants: 15 (11%) former patients or family members, 91 (68%) clinicians, and 28 (21%) researchers (for demographic characteristics, see Table 2). Of the 32 outcomes provided, 13 (40%) met the a priori criterion for COS inclusion (Table E2, http://links.lww.com/CCM/H556). In this phase, participants suggested 70 additional outcomes. Following review by the steering committee applying the selection criteria, 9 of these were included as outcomes for Round 2 (Table E3, http://links.lww.com/CCM/H556).
TABLE 2.
Participant Characteristics
| Patient and family interviews (N = 15) | n (%) |
| Patient | 10 (66) |
| Family | 5 (33) |
| Delphi (N = 134) | n (%) |
| Gender (male) | 31 (23) |
| Age (yr) | |
| < 30 | 8 (6) |
| 30–50 | 87 (65) |
| > 50 | 39 (29) |
| Country/continent of residence | |
| Canada | 11 (8) |
| Europe | 17 (13) |
| Australia or New Zealand | 45 (34) |
| United Kingdom | 29 (22) |
| United States | 25 (19) |
| Asia | 6 (4) |
| Other | 1 (1) |
| Involvement with people requiring a breathing tube | |
| Person/family member/friend | 15 (11) |
| Clinician | 91 (68) |
| Researcher | 28 (21) |
| Profession of healthcare participantsa | |
| Physician | 20 (17) |
| Nurse or nurse practitioner | 26 (22) |
| Speech Language Therapist | 58 (49) |
| Physical; occupational; or respiratory therapist | 14 (12) |
| Years of experience working with people with a breathing tubea | |
| 2–5 yr | 16 (14) |
| 6–10 yr | 19 (16) |
| > 10 yr | 83 (70) |
| Consensus meetings (N = 24) | n (%) |
| Patient and family | 3 (13) |
| Clinician | 14 (58) |
| Researcher | 7 (29) |
Data complete for 118 participants.
In total, 106 (81%) of the round 1 participants took part in round 2: 9 (8%) former patients or family members, 73 (69%) clinicians, and 24 (22%) researchers. Of the 41 outcomes provided in round 2, 22 (54%) met COS inclusion criteria (Table 3; for scores across all outcomes and groups, see Table E4, http://links.lww.com/CCM/H556). We identified scoring differences across the stakeholder groups for three outcomes; with only the patient and family group rating speech intelligibility as critical for inclusion, and only the clinician group rating use of physical restraint and fatigue related to the communication intervention as critical for inclusion.
TABLE 3.
Round 2 Delphi Scores Meeting Core Outcome Set Inclusion Criteria
| Outcomes—Overall Participants (N = 106) | Median (Interquartile Range) | Critical (%) |
|---|---|---|
| Ability of the patient to communicate when he/she wants to (i.e., availability of aid/device) | 8 (7–9) | 97 |
| Ability to communicate care; comfort; and safety needs | 9 (8–9) | 99 |
| Ability to gain attention (of care team/family member) | 9 (7–9) | 93 |
| Ability to participate in and direct a conversation (i.e., two-way exchange) | 8 (7–9) | 91 |
| Ability to communicate to participate in care decision-making | 9 (8–9) | 97 |
| Ability to use the provided communication aid/device | 9 (8–9) | 96 |
| Communication ease/effort | 7 (7–8) | 76 |
| Barriers and facilitators to communication | 7 (7–8) | 75 |
| Ability to communicate in a manner that means intentions and needs are understood by communication partner(s) | 8 (7–9) | 92 |
| Pain or discomfort related to use of a communication intervention | 7.5 (7–8) | 82 |
| Negative emotions associated with inability to communicate | 8 (7–9) | 90 |
| Positive emotions associated with ability to communicate | 8 (7–9) | 81 |
| Satisfaction with communication intervention | 8 (7–9) | 92 |
| Acceptability of the communication intervention | 8 (7–9) | 87 |
| Health-related quality of life | 8 (7–9) | 89 |
| Delirium prevalence | 7 (7–8) | 78 |
| Presence of agitation | 7 (6–7) | 78 |
| Mortality | 8 (7–9) | 75 |
| Adverse events as a result of communication interventiona | 8 (7–9) | 91 |
| Emotional wellbeing—this is referring to patient; family and healthcare providera | 7 (7–8) | 83 |
| Time to phonation; intelligible speech or functional communicationa | 7 (6–8) | 70 |
| Ability to develop and maintain relationships with healthcare providers; family and friendsa | 7 (7–8) | 87 |
Eighty-two participants (56 clinicians, 7 patient/family, 19 researchers) voted on this outcome in round 2.
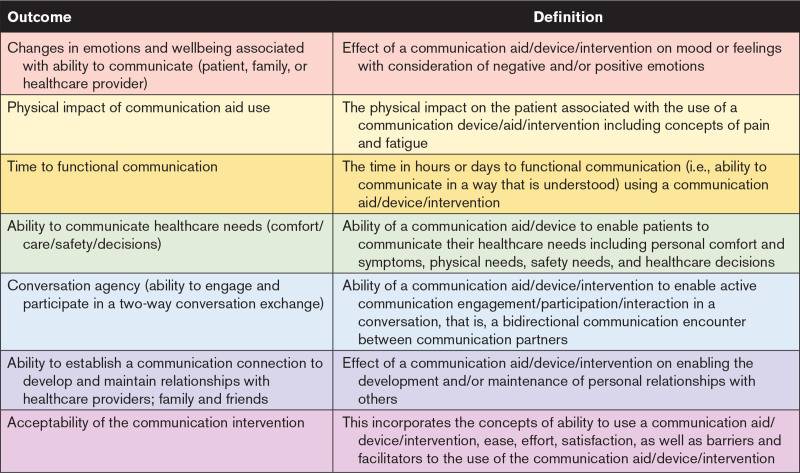
In total, 24 participants (3 [13%] patients, 14 [58%] clinicians, and 7 [29%] researchers) attended consensus meetings. Of the 22 outcomes presented, 4 were excluded: mortality, prevalence of delirium, presence of agitation, and health-related quality of life. Across the consensus meetings, there were robust discussions and agreement that while these outcomes are important to measure in a critical care population generally, as outcomes they should not be included in the minimum outcomes in studies evaluating the effect of a communication intervention. Other outcomes were grouped together (e.g., negative emotions, positive emotions and wellbeing were combined to form changes in emotion and wellbeing associated with ability to communicate). Final voting on seven outcomes by 19 of 24 consensus meeting participants (patient 16%, clinician 63% and researchers 21%) resulted in near total agreement 18 of 19 (94.6%) in the seven outcomes to include in the Comm-COS (Fig. 1). The final Comm-COS included: 1) changes in emotions and wellbeing associated with ability to communicate (patient, family or healthcare provider), 2) physical impact of communication aid use (e.g., pain, fatigue), 3) time to functional communication, 4) ability to communicate healthcare needs (comfort/care/safety/decisions), 5) conversation agency (ability to engage and participate in a two-way conversation exchange), 6) ability to establish a communication connection to develop and maintain relationships with healthcare providers; family and friends, and 7) acceptability of the communication intervention (concepts of ability to use, ease, effort, satisfaction, barriers, and facilitators). Expanded definitions of the Comm-COS are outlined in Table 4.
TABLE 4.
Final Communication Core Outcome Set With Definitions
DISCUSSION
This is one of the first studies to establish a COS specific to interventions promoting communication for critically ill adults with an artificial airway, with and without mechanical ventilation. Enabling and empowering patients to effectively communicate is a basic human right (31), and indeed a pillar of the humanization of care in ICU (32) and is often included as pivotal in enhancing safety standards for hospitals globally (33, 34). Seven outcomes achieved final consensus including: 1) changes in emotions and wellbeing associated with ability to communicate, 2) physical impact of communication aid use, 3) time to functional communication, 4) ability to communicate healthcare needs (comfort/care/safety/decisions), 5) conversation agency, that is, ability to engage and participate in a two-way communication exchange, 6) ability to establish a communication connection to develop and maintain relationships with healthcare providers, and 7) acceptability of the communication intervention (includes concepts relating to ability to use, ease, effort, satisfaction, barriers, and facilitators). These outcomes reflect the dynamic and complex elements of communication that span across body structure, activities, and participation elements of the International Classification of Functioning, Disability and Health (35).
Following COMET guidance and oversight by a multidisciplinary and patient steering committee, we generated Delphi outcomes from a systematic review and qualitative interviews with patients and families with lived experience. The generation of outcomes from lived experience in critical care is crucial as these interventions are delivered to patients, and it is vital to consider what is important to them (36). The Comm-COS considers the dynamic nature of communication with the inclusion of outcomes relevant to the content, purpose, and wider personal goals (e.g., establishing social connection and sense of identity) of communication. The highest ranked outcome across all stakeholder groups was the ability to communicate healthcare needs (i.e., comfort/care/safety/decisions). This outcome aligns with previously published evidence reporting diverse communication needs of critically ill patients with an artificial airway (11, 13, 37, 38) and the need for patient communication that is understood by communication partners. Additionally, outcomes pertaining to a sense of personal connection and wellbeing were recognized as core. This affirms the role of effective communication with a sense of identity, increased engagement in healthcare, and concept of recovery (11, 39).
During the final iterative consensus processes, several outcomes were condensed including “ability to use the provided communication aid,” “communication ease/effort,” and “satisfaction with communication” into acceptability of the communication intervention as these concepts were considered under the broader construct of acceptability (Table 4) (40). Similarly, outcomes describing either positive or negative emotions were condensed. During the consensus discussions, participants acknowledged emotions occur along a continuum and nominating only a singular positive or negative emotion associated with enabling communication was too restrictive. This resulted in the outcome changes in emotions and wellbeing associated with ability to communicate. Although there was some variation in the discussion content at consensus meetings, there were similarities regarding groupings/combination of outcomes across the consensus meetings. For future trials enabling communication, researchers are not restricted to using only outcomes nominated for the Comm-COS. Researchers can use additional outcomes relevant to their intervention. These may include process (e.g., documentation of communication method), economic (e.g., cost), cognitive status (e.g., delirium), or implementation (e.g., number of intervention sessions delivered) outcomes.
The Comm-COS is suitable for future trials of interventions enabling communication for critically ill patients with an artificial airway including both endotracheal and tracheostomy tubes. Widespread use of the Comm-COS has the potential to optimize comparison of interventions with outcomes that are relevant to all stakeholders. Although the guidance of what to measure has been established, the next stage we will undertake is how to measure these outcomes with consideration of tools, timing of measurement, and who they should be collected from (i.e., patient, family members, and healthcare providers) (41). Until this second step is completed, researchers should use the Comm-COS outcomes and select measures considered relevant to their needs as is done with current study designs. During COS development phase, lack of consensus on the use of existing measurement tools to detect meaningful change is common (42, 43).
Based on our preliminary measurement work, we suspect that there are no current valid and reliable measures for outcomes such as conversational agency and ability to establish a communication connection to develop and maintain relationships with healthcare providers. Indeed, given the lack of validated measures currently used in intervention studies, there is an urgent need to develop specific and sensitive measurement tools of patient communication in critical care settings that consider body systems, function, and participation (17). The recently developed Communication with an Artificial airway Tool has elements (e.g., comprehension: basic and complex information and communication output: content and clarity) that align with conversational agency and ability to communicate healthcare needs outcomes in the Comm-COS; however, further validation is required (44). Measures validated in other populations to assess changes in positive and negative emotions may be suitable measures of the Comm-COS. The Visual Analogue Scale of Self-Esteem measures change in 10 domains of self-esteem and emotion that include both positive and negative emotions (e.g., depression, optimism, frustration, and cheerfulness) which aligns with the outcome changes in emotions and wellbeing associated with ability to communicate (45). Similarly, the Positive and Negative Affect Scales is a reliable and valid measure of 10 elements of different mood dimensions and their contribution to overall affect (46).
Strengths of this study are the rigorous multistage methods, international stakeholder participation, and governance provided by the ESC (47). As our COS was endorsed by two critical care professional societies and one ICU consumer organization, multiple recruitment pathways were used to optimize international engagement. However, stakeholder participation was limited to those that could speak English, participate in electronic voting, and attend virtual consensus meetings which favored participation from people in minority-world countries. Some participant attrition occurred across the Delphi Rounds; however, we were able to retain participants in all three stakeholder groups including the final consensus voting. Although there was consumer representation and participation across all Delphi phases, we acknowledge that this proportion was low. One reason for this reported by our participants was an inability to remain engaged over the entire Delphi process due to the time commitment and physical health. Comm-Cos was developed for critically ill adults with an artificial airway therefore further COS development may be required for other patients including those in rehabilitation centers and community settings. Despite further work required to determine the measures for the Comm-COS, determining which outcomes are meaningful from all stakeholders’ perspectives is an important step informing future intervention trials.
CONCLUSIONS
The Comm-COS comprises of seven outcomes for evaluating interventions enabling communication for critically ill adults with an artificial airway. These outcomes can now guide the development and validation of relevant patient communication measures for the Comm-COS. The adoption of the Comm-COS in future studies will help establish comparable trials in the pursuit of effective interventions to ameliorate the highly prevalent and negative experience of communicative incapacity.
ACKNOWLEDGMENTS
The authors acknowledge Mariyam Ali for her assistance with data extraction for the systematic review. The authors also acknowledge all consensus panel participants: Tami Altschuler, Lawrence Caruana, Nicola Clayton, Gemma Clunie, Brian Cuthbertson, Marleen Flim, David Garfield, Mohan Gurjar, Tim Higgins, Anne Højager Nielsen, Richard Huff, Marta Kazandjian, Kristin King, Andrea Marshall, Kate McCleary, Jackie McRae, Clare Mills, Helen Newman, Lance Patak, Rachel Santiago, Sanjay Singh, Pieter Tuinman, Sarah Wallace OBE, and Marte-Marie Wallander. This study was formally endorsed by the U.K. Intensive Care Society, the European Society of Intensive Care Medicine, and ICUsteps.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The research leading to these results received seed funding from the Graduate School of Health, Faculty of Health, University of Technology Sydney.
Dr. Freeman-Sanderson was involved in conceptualization, funding acquisition, methodology, data curation, formal analysis, writing the original draft, visualization, and project administration. Dr. Rose was involved in conceptualization, methodology, data curation, formal analysis, supervision, and writing the original draft, reviewing, and editing. Ms. Sullivan was involved in data curation, visualization, and reviewing and editing the writing. The Expert Advisory Committee (Drs. Brodsky, Dale, Gupta, Haines, Happ, Hart, Hemsley, Istanboulian, Spronk, and Sutt) was involved in conceptualization, data curation (Drs. Sutt and Istanboulian), analysis, and reviewing and editing the writing. All authors contributed to the design, writing, and editing of the article.
Open Science Framework Registration: https://osf.io/pkzy9.
Core Outcome Measures in Effectiveness Trials registration: http://www.comet-initiative.org/Studies/Details/1671.
Dr. Freeman-Sanderson received support for article research from an internal university seed grant from Faculty of Health, University of Technology Sydney (UTS). Dr. Brodsky’s institution received funding from the National Institutes of Health and the Food and Drug Administration; he received funding from MedBridge and Phagenesis. Dr. Happ received funding from internal research funds, the Healthy State Alliance Momentum grant; she disclosed that she holds the copyright to the Study of Patient-Nurse Effectiveness with Assisted Communication Strategies (SPEACS-2) online communication training program. Sullivan’s institution received funding from UTS. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Martin B. Brodsky, Email: brodskm@ccf.org.
Craig Dale, Email: craig.dale@utoronto.ca.
Anushua Gupta, Email: anushua.gupta@nhs.net.
Kimberley Haines, Email: Kimberley.Haines@wh.org.au.
Mary Beth Happ, Email: Happ.3@osu.edu.
Nicholas Hart, Email: Nicholas.Hart@gstt.nhs.uk.
Bronwyn Hemsley, Email: Bronwyn.Hemsley@uts.edu.au.
Laura Istanboulian, Email: laura.istanboulian@tehn.ca.
Peter Spronk, Email: p.spronk@gelre.nl.
Rebecca Sullivan, Email: Rebecca.Sullivan-1@student.uts.edu.au.
Anna-Liisa Sutt, Email: annaliisasp@gmail.com.
Louise Rose, Email: louise.rose@kcl.ac.uk.
REFERENCES
- 1.ten Hoorn S, Elbers PW, Girbes AR, et al. : Communicating with conscious and mechanically ventilated critically ill patients: A systematic review. Crit Care. 2016; 20:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose L, Istanboulian L, Smith OM, et al. : Feasibility of the electrolarynx for enabling communication in the chronically critically ill: The EECCHO study. J Crit Care. 2018; 47:109–113 [DOI] [PubMed] [Google Scholar]
- 3.Zaga CJ, Freeman-Sanderson A, Happ MB, et al. : Defining effective communication for critically ill patients with an artificial airway: An international multi-professional consensus. Intensive Crit Care Nurs. 2023; 76:103393. [DOI] [PubMed] [Google Scholar]
- 4.Herridge MS, Azoulay E: Outcomes after critical illness. N Engl J Med. 2023; 388:913–924 [DOI] [PubMed] [Google Scholar]
- 5.Wilcox ME, Brummel NE, Archer K, et al. : Cognitive dysfunction in ICU patients: Risk factors, predictors, and rehabilitation interventions. Crit Care Med. 2013; 41(9 Suppl 1):S81–S98 [DOI] [PubMed] [Google Scholar]
- 6.Guttormson J, Bremer K, Jones R: “Not being able to talk was horrid”: A descriptive, correlational study of communication during mechanical ventilation. Intensive Crit Care Nurs. 2015; 31:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Istanboulian L, Rose L, Gorospe F, et al. : Barriers to and facilitators for the use of augmentative and alternative communication and voice restorative strategies for adults with an advanced airway in the intensive care unit: A scoping review. J Crit Care. 2020; 57:168–176 [DOI] [PubMed] [Google Scholar]
- 8.Freeman-Sanderson AL, Togher L, Elkins MR, et al. : Quality of life improves with return of voice in tracheostomy patients in intensive care: An observational study. J Crit Care. 2016; 33:186–191 [DOI] [PubMed] [Google Scholar]
- 9.Freeman-Sanderson AL, Togher L, Elkins MR, et al. : Return of voice for ventilated tracheostomy patients in ICU: A randomized controlled trial of early-targeted intervention. Crit Care Med. 2016; 44:1075–1081 [DOI] [PubMed] [Google Scholar]
- 10.Huttmann SE, Magnet FS, Karagiannidis C, et al. : Quality of life and life satisfaction are severely impaired in patients with long-term invasive ventilation following ICU treatment and unsuccessful weaning. Ann Intensive Care. 2018; 8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman-Sanderson A, Togher L, Elkins M, et al. : Quality of life improves for tracheostomy patients with return of voice: A mixed methods evaluation of the patient experience across the care continuum. Intensive Crit Care Nurs. 2018; 46:10–16 [DOI] [PubMed] [Google Scholar]
- 12.Nyhagen R, Egerod I, Rustøen T, et al. : Unidentified communication challenges in the intensive care unit: A qualitative study using multiple triangulations. Aust Crit Care. 2022; 36:215–222 [DOI] [PubMed] [Google Scholar]
- 13.Karlsen MMW, Happ MB, Finset A, et al. : Patient involvement in micro-decisions in intensive care. Patient Educ Couns. 2020; 103:2252–2259 [DOI] [PubMed] [Google Scholar]
- 14.Guttormson JL, Khan B, Brodsky MB, et al. : Symptom assessment for mechanically ventilated patients: Principles and priorities: an official American thoracic society workshop report. Ann Am Thorac Soc. 2023; 20:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson C, Clunie G, Evison F, et al. ; PHOSP-COVID collaborative Group: Prevalence of swallow, communication, voice and cognitive compromise following hospitalisation for COVID-19: The PHOSP-COVID analysis. BMJ Open Respir Res. 2023; 10:e001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carruthers H, Astin F, Munro W: Which alternative communication methods are effective for voiceless patients in intensive care units? A systematic review. Intensive Crit Care Nurs. 2017; 42:88–96 [DOI] [PubMed] [Google Scholar]
- 17.Rose L, Sutt AL, Amaral AC, et al. : Interventions to enable communication for adult patients requiring an artificial airway with or without mechanical ventilator support. Cochrane Database Syst Rev. 2021; 10:CD013379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall JC, Vincent JL, Guyatt G, et al. : Outcome measures for clinical research in sepsis: A report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005; 33:1708–1716 [DOI] [PubMed] [Google Scholar]
- 19.Sanderson T, Morris M, Calnan M, et al. : What outcomes from pharmacologic treatments are important to people with rheumatoid arthritis? Creating the basis of a patient core set. Arthritis Care Res. 2010; 62:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinglas VD, Cherukuri SPS, Needham DM: Core outcomes sets for studies evaluating critical illness and patient recovery. Curr Opin Crit Care. 2020; 26:489–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson PR, Altman DG, Bagley H, et al. : The COMET handbook: Version 1.0. Trials. 2017; 18(Suppl 3):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkham JJ, Gorst S, Altman DG, et al. : Core outcome set—STAndards for reporting: The COS-STAR statement. PLoS Med. 2016; 13:e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palinkas LA, Horwitz SM, Green CA, et al. : Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015; 42:533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elo S, Kyngas H: The qualitative content analysis process. J Adv Nurs. 2008; 62:107–115 [DOI] [PubMed] [Google Scholar]
- 26.Dodd S, Clarke M, Becker L, et al. : A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018; 96:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillan SS, King M, Tully MP: How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016; 38:655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mease PJ, Clauw DJ, Arnold LM, et al. : Fibromyalgia syndrome. J Rheumatol. 2005; 32:2270–2277 [PubMed] [Google Scholar]
- 30.Mease P, Arnold LM, Bennett R, et al. : Fibromyalgia syndrome. J Rheumatol. 2007; 34:1415–1425 [PubMed] [Google Scholar]
- 31.McLeod S: Communication rights: Fundamental human rights for all. Int J Speech Lang Pathol. 2018; 20:3–11 [DOI] [PubMed] [Google Scholar]
- 32.Nin Vaeza N, Martin Delgado MC, Heras La Calle G: Humanizing intensive care: Toward a human-centered care ICU model. Crit Care Med. 2020; 48:385–390 [DOI] [PubMed] [Google Scholar]
- 33.NHS England: Improving patient safety culture—a practical guide. Available at: https://www.england.nhs.uk/publication/improving-patient-safety-culture-a-practical-guide/. Accessed October 18, 2023 [Google Scholar]
- 34.Australian Commission on Safety and Quality in Health Care: National Safety and Quality Health Service Standards: Guide for Hospitals. Available at: https://www.safetyandquality.gov.au/sites/default/files/2019-05/national-safety-and-quality-health-service-standards-guide-for-hospitals.pdf. Accessed October 18, 2023 [Google Scholar]
- 35.World Health Organization: ICF: International Classification of Functioning, Disability and Health. Geneva, Switzerland, WHO, 2001 [Google Scholar]
- 36.Dinglas VD, Faraone LN, Needham DM: Understanding patient-important outcomes after critical illness: A synthesis of recent qualitative, empirical, and consensus-related studies. Curr Opin Crit Care. 2018; 24:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallander Karlsen M-M, Heggdal K, Finset A, et al. : Attention-seeking actions by patients on mechanical ventilation in intensive care units: A phenomenological-hermeneutical study. J Clin Nurs. 2019; 28:66–79 [DOI] [PubMed] [Google Scholar]
- 38.Happ MB, Swigart VA, Tate JA, et al. : Patient involvement in health-related decisions during prolonged critical illness. Res Nurs Health. 2007; 30:361–372 [DOI] [PubMed] [Google Scholar]
- 39.Newman H, Clunie G, Wallace S, et al. : What matters most to adults with a tracheostomy in ICU and the implications for clinical practice: A qualitative systematic review and metasynthesis. J Crit Care. 2022; 72:154145. [DOI] [PubMed] [Google Scholar]
- 40.Sekhon M, Cartwright M, Francis JJ: Acceptability of healthcare interventions: An overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017; 17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prinsen CA, Vohra S, Rose MR, et al. : How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”—a practical guideline. Trials. 2016; 17:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose L, Burry L, Agar M, et al. ; Del-COrS Group: A core outcome set for research evaluating interventions to prevent and/or treat delirium in critically ill adults: An International Consensus Study (Del-COrS). Crit Care Med. 2021; 49:1535–1546 [DOI] [PubMed] [Google Scholar]
- 43.Munblit D, Nicholson T, Akrami A, et al. ; PC-COS project steering committee: A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: An international Delphi consensus study. Lancet Respir Med. 2022; 10:715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaga CJ, Papasavva CS, Hepworth G, et al. : Development, feasibility testing, and preliminary evaluation of the Communication with an Artificial airway Tool (CAT): Results of the Crit-CAT pilot study. Aust Crit Care. 2024; 37:127–137 [DOI] [PubMed] [Google Scholar]
- 45.Brumfitt SM, Sheeran P: The development and validation of the Visual Analogue Self-Esteem Scale (VASES). Br J Clin Psychol. 1999; 38:387–400 [DOI] [PubMed] [Google Scholar]
- 46.Watson D, Clark LA, Tellegen A: Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988; 54:1063–1070 [DOI] [PubMed] [Google Scholar]
- 47.Brookes ST, Macefield RC, Williamson PR, et al. : Three nested randomized controlled trials of peer-only or multiple stakeholder group feedback within Delphi surveys during core outcome and information set development. Trials. 2016; 17:409. [DOI] [PMC free article] [PubMed] [Google Scholar]