Abstract
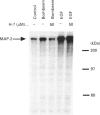
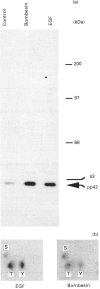
Both bombesin and epidermal growth factor (EGF) are potent mitogens in Swiss 3T3 cells that nonetheless have dissimilar receptor structures. To explore possible common intracellular events involved in the stimulation of cellular growth by these two peptides, we have evaluated the regulation of the mitogen-activated protein (MAP) kinase. Exposure of Swiss 3T3 cells to bombesin, EGF or the protein kinase C activator phorbol 12-myristate 13-acetate (PMA) causes the rapid and transient stimulation of the enzyme activity. Pretreatment of cells with the protein kinase inhibitor H-7, or down-regulation of cellular protein kinase C by prolonged exposure to PMA, causes a decrease of over 90% in the activation of MAP kinase by bombesin. In contrast, these treatments have no effect on the stimulation of MAP kinase by EGF. The stimulation of MAP kinase activity by bombesin is dose-dependent, occurring over a narrow concentration range of the peptide. Both EGF and bombesin stimulate the phosphorylation of an immunoprecipitable MAP kinase protein migrating at 42 kDa on SDS/PAGE. Phosphoamino acid analysis of this phosphorylated protein reveals that EGF and bombesin stimulate phosphorylation on tyrosine, threonine and serine residues. Tyrosine phosphorylation of the enzyme, as evaluated by antiphosphotyrosine blotting of the immunoprecipitated protein, reveals that the time course of phosphorylation by both mitogens correlates with stimulation of enzyme activity. These results provide further evidence for the convergence of discrete pathways emanating from tyrosine kinase and G-protein-linked receptors in the regulation of MAP kinase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. D., Parker P. J. TPA-induced activation of MAP kinase. FEBS Lett. 1991 Sep 23;290(1-2):77–82. doi: 10.1016/0014-5793(91)81230-6. [DOI] [PubMed] [Google Scholar]
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Anderson N. G., Kilgour E., Sturgill T. W. Activation of mitogen-activated protein kinase in BC3H1 myocytes by fluoroaluminate. J Biol Chem. 1991 Jun 5;266(16):10131–10135. [PubMed] [Google Scholar]
- Anderson N. G., Li P., Marsden L. A., Williams N., Roberts T. M., Sturgill T. W. Raf-1 is a potential substrate for mitogen-activated protein kinase in vivo. Biochem J. 1991 Jul 15;277(Pt 2):573–576. doi: 10.1042/bj2770573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Bading H., Greenberg M. E. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991 Aug 23;253(5022):912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Battey J. F., Way J. M., Corjay M. H., Shapira H., Kusano K., Harkins R., Wu J. M., Slattery T., Mann E., Feldman R. I. Molecular cloning of the bombesin/gastrin-releasing peptide receptor from Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):395–399. doi: 10.1073/pnas.88.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. A., Sleath P. R., deRoos P. C., Dower S. K., Virca G. D. Interleukin-1 represents a new modality for the activation of extracellular signal-regulated kinases/microtubule-associated protein-2 kinases. J Biol Chem. 1991 Nov 25;266(33):22661–22670. [PubMed] [Google Scholar]
- Boulton T. G., Cobb M. H. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991 May;2(5):357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Gregory J. S., Cobb M. H. Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimulated microtubule-associated protein 2 kinase. Biochemistry. 1991 Jan 8;30(1):278–286. doi: 10.1021/bi00215a038. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Cahill A. L., Perlman R. L. Activation of a microtubule-associated protein-2 kinase by insulin-like growth factor-I in bovine chromaffin cells. J Neurochem. 1991 Dec;57(6):1832–1839. doi: 10.1111/j.1471-4159.1991.tb06392.x. [DOI] [PubMed] [Google Scholar]
- Campbell G. S., Pang L., Miyasaka T., Saltiel A. R., Carter-Su C. Stimulation by growth hormone of MAP kinase activity in 3T3-F442A fibroblasts. J Biol Chem. 1992 Mar 25;267(9):6074–6080. [PubMed] [Google Scholar]
- Carpenter C. D., Ingraham H. A., Cochet C., Walton G. M., Lazar C. S., Sowadski J. M., Rosenfeld M. G., Gill G. N. Structural analysis of the transmembrane domain of the epidermal growth factor receptor. J Biol Chem. 1991 Mar 25;266(9):5750–5755. [PubMed] [Google Scholar]
- Ely C. M., Oddie K. M., Litz J. S., Rossomando A. J., Kanner S. B., Sturgill T. W., Parsons S. J. A 42-kD tyrosine kinase substrate linked to chromaffin cell secretion exhibits an associated MAP kinase activity and is highly related to a 42-kD mitogen-stimulated protein in fibroblasts. J Cell Biol. 1990 Mar;110(3):731–742. doi: 10.1083/jcb.110.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force T., Kyriakis J. M., Avruch J., Bonventre J. V. Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and -independent pathways in glomerular mesangial cells. J Biol Chem. 1991 Apr 5;266(10):6650–6656. [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Sakai H. Okadaic acid activates microtubule-associated protein kinase in quiescent fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):671–674. doi: 10.1111/j.1432-1033.1990.tb19385.x. [DOI] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Gómez N., Tonks N. K., Morrison C., Harmar T., Cohen P. Evidence for communication between nerve growth factor and protein tyrosine phosphorylation. FEBS Lett. 1990 Oct 1;271(1-2):119–122. doi: 10.1016/0014-5793(90)80386-w. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Activation of a Ca2+-inhibitable protein kinase that phosphorylates microtubule-associated protein 2 in vitro by growth factors, phorbol esters, and serum in quiescent cultured human fibroblasts. J Biol Chem. 1988 Apr 15;263(11):5396–5401. [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Characterization of a mitogen-activated, Ca2+-sensitive microtubule-associated protein-2 kinase. Eur J Biochem. 1989 Sep 15;184(2):477–486. doi: 10.1111/j.1432-1033.1989.tb15040.x. [DOI] [PubMed] [Google Scholar]
- Huckle W. R., Prokop C. A., Dy R. C., Herman B., Earp S. Angiotensin II stimulates protein-tyrosine phosphorylation in a calcium-dependent manner. Mol Cell Biol. 1990 Dec;10(12):6290–6298. doi: 10.1128/mcb.10.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Allemain G., Sturgill T. W., Weber M. J. Defective regulation of mitogen-activated protein kinase activity in a 3T3 cell variant mitogenically nonresponsive to tetradecanoyl phorbol acetate. Mol Cell Biol. 1991 Feb;11(2):1002–1008. doi: 10.1128/mcb.11.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. M., Cobb M. H., Blackshear P. J. Evidence that extracellular signal-regulated kinases are the insulin-activated Raf-1 kinase kinases. J Biol Chem. 1992 Jan 15;267(2):1088–1092. [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Song X. H. Bradykinin and bombesin rapidly stimulate tyrosine phosphorylation of a 120-kDa group of proteins in Swiss 3T3 cells. J Biol Chem. 1991 Apr 25;266(12):7746–7749. [PubMed] [Google Scholar]
- Miyasaka T., Chao M. V., Sherline P., Saltiel A. R. Nerve growth factor stimulates a protein kinase in PC-12 cells that phosphorylates microtubule-associated protein-2. J Biol Chem. 1990 Mar 15;265(8):4730–4735. [PubMed] [Google Scholar]
- Miyasaka T., Sternberg D. W., Miyasaka J., Sherline P., Saltiel A. R. Nerve growth factor stimulates protein tyrosine phosphorylation in PC-12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2653–2657. doi: 10.1073/pnas.88.7.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Rheeder A., Williams K., Pollack S., Katz R., Landreth G. E. Stimulation of MAP-2 kinase activity in T lymphocytes by anti-CD3 or anti-Ti monoclonal antibody is partially dependent on protein kinase C. J Immunol. 1990 Apr 1;144(7):2683–2689. [PubMed] [Google Scholar]
- Ohmichi M., Decker S. J., Pang L., Saltiel A. R. Phospholipase C-gamma 1 directly associates with the p70 trk oncogene product through its src homology domains. J Biol Chem. 1991 Aug 15;266(23):14858–14861. [PubMed] [Google Scholar]
- Ohmichi M., Decker S. J., Saltiel A. R. Nerve growth factor induces the association of a 130-Kd phosphoprotein with its receptor in PC-12 pheochromocytoma cells. Cell Regul. 1991 Sep;2(9):691–697. doi: 10.1091/mbc.2.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. J Biol Chem. 1988 Sep 5;263(25):12721–12727. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Sanghera J. S., Marsden L. A., Weber M. J., Pelech S. L., Sturgill T. W. Biochemical characterization of a family of serine/threonine protein kinases regulated by tyrosine and serine/threonine phosphorylations. J Biol Chem. 1991 Oct 25;266(30):20270–20275. [PubMed] [Google Scholar]
- Rozengurt E., Fabregat I., Coffer A., Gil J., Sinnett-Smith J. Mitogenic signalling through the bombesin receptor: role of a guanine nucleotide regulatory protein. J Cell Sci Suppl. 1990;13:43–56. doi: 10.1242/jcs.1990.supplement_13.6. [DOI] [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Pelech S. L. Role of protein phosphorylation in the maturation-induced activation of a myelin basic protein kinase from sea star oocytes. J Biol Chem. 1991 Apr 15;266(11):6700–6707. [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Takishima K., Griswold-Prenner I., Ingebritsen T., Rosner M. R. Epidermal growth factor (EGF) receptor T669 peptide kinase from 3T3-L1 cells is an EGF-stimulated "MAP" kinase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2520–2524. doi: 10.1073/pnas.88.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Iwamoto A., Kumada M., Yamashita K., Takuwa Y. Role of Ca2+ influx in bombesin-induced mitogenesis in Swiss 3T3 fibroblasts. J Biol Chem. 1991 Jan 25;266(3):1403–1409. [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Tobe K., Kadowaki T., Tamemoto H., Ueki K., Hara K., Koshio O., Momomura K., Gotoh Y., Nishida E., Akanuma Y. Insulin and 12-O-tetradecanoylphorbol-13-acetate activation of two immunologically distinct myelin basic protein/microtubule-associated protein 2 (MBP/MAP2) kinases via de novo phosphorylation of threonine and tyrosine residues. J Biol Chem. 1991 Dec 25;266(36):24793–24803. [PubMed] [Google Scholar]
- Tsao H., Aletta J. M., Greene L. A. Nerve growth factor and fibroblast growth factor selectively activate a protein kinase that phosphorylates high molecular weight microtubule-associated proteins. Detection, partial purification, and characterization in PC12 cells. J Biol Chem. 1990 Sep 15;265(26):15471–15480. [PubMed] [Google Scholar]
- Wu J., Rossomando A. J., Her J. H., Del Vecchio R., Weber M. J., Sturgill T. W. Autophosphorylation in vitro of recombinant 42-kilodalton mitogen-activated protein kinase on tyrosine. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9508–9512. doi: 10.1073/pnas.88.21.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]