Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildgaard U. Highly purified antithrombin 3 with heparin cofactor activity prepared by disc electrophoresis. Scand J Clin Lab Invest. 1968;21(1):89–91. doi: 10.3109/00365516809076981. [DOI] [PubMed] [Google Scholar]
- Abildgaard U. Tissue factor pathway inhibitor and heparin. Adv Exp Med Biol. 1992;313:199–204. doi: 10.1007/978-1-4899-2444-5_20. [DOI] [PubMed] [Google Scholar]
- Akiyama F., Seno N., Yoshida K. Anticoagulant activity of dermatan polysulfates. Tohoku J Exp Med. 1982 Apr;136(4):359–365. doi: 10.1620/tjem.136.359. [DOI] [PubMed] [Google Scholar]
- Andersson L. O., Barrowcliffe T. W., Holmer E., Johnson E. A., Sims G. E. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin iii and by gel filtration. Thromb Res. 1976 Dec;9(6):575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- Andersson T. R., Larsen M. L., Abildgaard U. Low heparin cofactor II associated with abnormal crossed immunoelectrophoresis pattern in two Norwegian families. Thromb Res. 1987 Jul 15;47(2):243–248. doi: 10.1016/0049-3848(87)90381-1. [DOI] [PubMed] [Google Scholar]
- Asakura S., Hirata H., Okazaki H., Hashimoto-Gotoh T., Matsuda M. Hydrophobic residues 382-386 of antithrombin III, Ala-Ala-Ala-Ser-Thr, serve as the epitope for an antibody which facilitates hydrolysis of the inhibitor by thrombin. J Biol Chem. 1990 Mar 25;265(9):5135–5138. [PubMed] [Google Scholar]
- Atha D. H., Lormeau J. C., Petitou M., Rosenberg R. D., Choay J. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry. 1985 Nov 5;24(23):6723–6729. doi: 10.1021/bi00344a063. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Stephens A. W., Rosenberg R. D. Evaluation of critical groups required for the binding of heparin to antithrombin. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1030–1034. doi: 10.1073/pnas.81.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Barrowcliffe T. W., Merton R. E., Havercroft S. J., Thunberg L., Lindahl U., Thomas D. P. Low-affinity heparin potentiates the action of high-affinity heparin oligosaccharides. Thromb Res. 1984 Apr 15;34(2):125–133. doi: 10.1016/0049-3848(84)90069-0. [DOI] [PubMed] [Google Scholar]
- Beeler D., Rosenberg R., Jordan R. Fractionation of low molecular weight heparin species and their interaction with antithrombin. J Biol Chem. 1979 Apr 25;254(8):2902–2913. [PubMed] [Google Scholar]
- Beresford C. H., Owen M. C. Antithrombin III. Int J Biochem. 1990;22(2):121–128. doi: 10.1016/0020-711x(90)90172-y. [DOI] [PubMed] [Google Scholar]
- Bertina R. M., van der Linden I. K., Engesser L., Muller H. P., Brommer E. J. Hereditary heparin cofactor II deficiency and the risk of development of thrombosis. Thromb Haemost. 1987 Apr 7;57(2):196–200. [PubMed] [Google Scholar]
- Bienkowski M. J., Conrad H. E. Structural characterization of the oligosaccharides formed by depolymerization of heparin with nitrous acid. J Biol Chem. 1985 Jan 10;260(1):356–365. [PubMed] [Google Scholar]
- Björk I., Fish W. W. Production in vitro and properties of a modified form of bovine antithrombin, cleaved at the active site by thrombin. J Biol Chem. 1982 Aug 25;257(16):9487–9493. [PubMed] [Google Scholar]
- Björk I., Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982 Oct 29;48(3):161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- Björk I., Nordenman B. Acceleration of the reaction between thrombin and antithrombin III by non-stoichiometric amounts of heparin. Eur J Biochem. 1976 Sep 15;68(2):507–511. doi: 10.1111/j.1432-1033.1976.tb10838.x. [DOI] [PubMed] [Google Scholar]
- Björk I., Olson S. T., Sheffer R. G., Shore J. D. Binding of heparin to human high molecular weight kininogen. Biochemistry. 1989 Feb 7;28(3):1213–1221. doi: 10.1021/bi00429a039. [DOI] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K., Olson S. T., Bock P. E. Conversion of antithrombin from an inhibitor of thrombin to a substrate with reduced heparin affinity and enhanced conformational stability by binding of a tetradecapeptide corresponding to the P1 to P14 region of the putative reactive bond loop of the inhibitor. J Biol Chem. 1992 Jan 25;267(3):1976–1982. [PubMed] [Google Scholar]
- Blackburn M. N., Smith R. L., Carson J., Sibley C. C. The heparin-binding site of antithrombin III. Identification of a critical tryptophan in the amino acid sequence. J Biol Chem. 1984 Jan 25;259(2):939–941. [PubMed] [Google Scholar]
- Blinder M. A., Andersson T. R., Abildgaard U., Tollefsen D. M. Heparin cofactor IIOslo. Mutation of Arg-189 to His decreases the affinity for dermatan sulfate. J Biol Chem. 1989 Mar 25;264(9):5128–5133. [PubMed] [Google Scholar]
- Blinder M. A., Marasa J. C., Reynolds C. H., Deaven L. L., Tollefsen D. M. Heparin cofactor II: cDNA sequence, chromosome localization, restriction fragment length polymorphism, and expression in Escherichia coli. Biochemistry. 1988 Jan 26;27(2):752–759. doi: 10.1021/bi00402a039. [DOI] [PubMed] [Google Scholar]
- Blinder M. A., Tollefsen D. M. Site-directed mutagenesis of arginine 103 and lysine 185 in the proposed glycosaminoglycan-binding site of heparin cofactor II. J Biol Chem. 1990 Jan 5;265(1):286–291. [PubMed] [Google Scholar]
- Boffa M. C., Burke B., Haudenschild C. C. Preservation of thrombomodulin antigen on vascular and extravascular surfaces. J Histochem Cytochem. 1987 Nov;35(11):1267–1276. doi: 10.1177/35.11.2821107. [DOI] [PubMed] [Google Scholar]
- Borg J. Y., Brennan S. O., Carrell R. W., George P., Perry D. J., Shaw J. Antithrombin Rouen-IV 24 Arg----Cys. The amino-terminal contribution to heparin binding. FEBS Lett. 1990 Jun 18;266(1-2):163–166. doi: 10.1016/0014-5793(90)81530-2. [DOI] [PubMed] [Google Scholar]
- Bourdon M. A., Krusius T., Campbell S., Schwartz N. B., Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
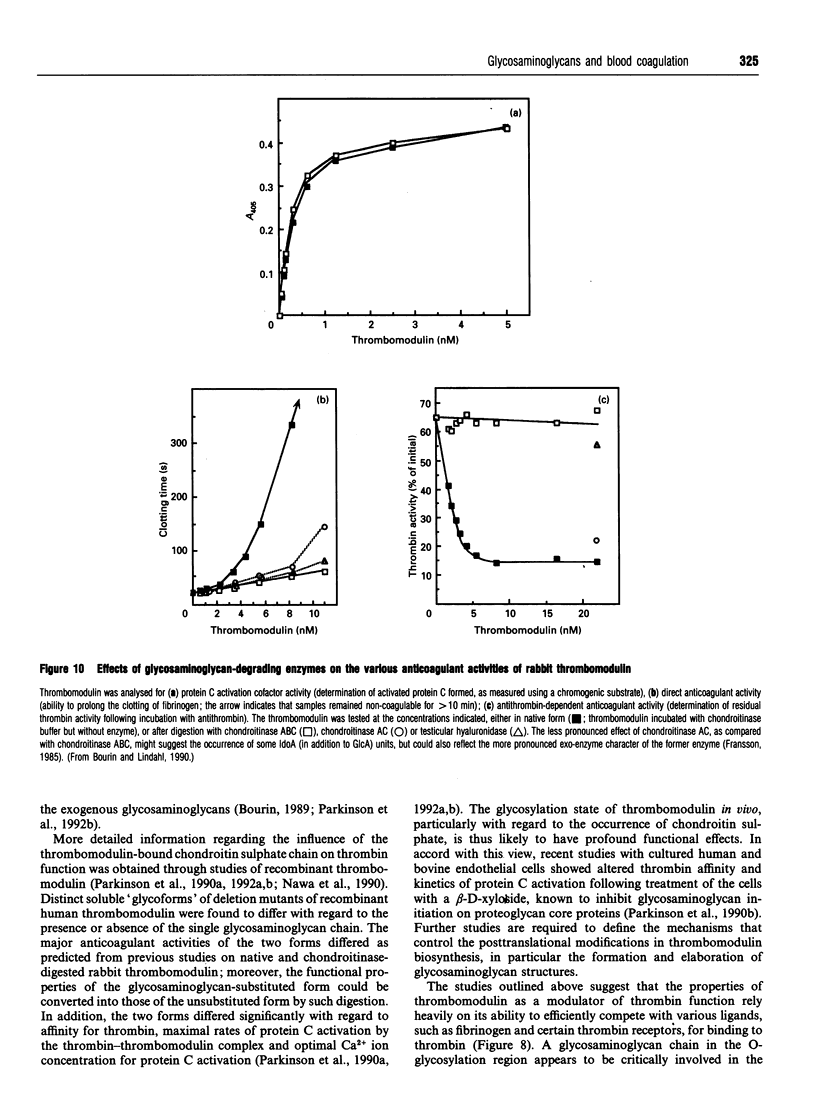
- Bourin M. C., Boffa M. C., Björk I., Lindahl U. Functional domains of rabbit thrombomodulin. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5924–5928. doi: 10.1073/pnas.83.16.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M. C. Effect of rabbit thrombomodulin on thrombin inhibition by antithrombin in the presence of heparin. Thromb Res. 1989 Apr 1;54(1):27–39. doi: 10.1016/0049-3848(89)90334-4. [DOI] [PubMed] [Google Scholar]
- Bourin M. C., Lindahl U. Functional role of the polysaccharide component of rabbit thrombomodulin proteoglycan. Effects on inactivation of thrombin by antithrombin, cleavage of fibrinogen by thrombin and thrombin-catalysed activation of factor V. Biochem J. 1990 Sep 1;270(2):419–425. doi: 10.1042/bj2700419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M. C., Lundgren-Akerlund E., Lindahl U. Isolation and characterization of the glycosaminoglycan component of rabbit thrombomodulin proteoglycan. J Biol Chem. 1990 Sep 15;265(26):15424–15431. [PubMed] [Google Scholar]
- Bourin M. C., Ohlin A. K., Lane D. A., Stenflo J., Lindahl U. Relationship between anticoagulant activities and polyanionic properties of rabbit thrombomodulin. J Biol Chem. 1988 Jun 15;263(17):8044–8052. [PubMed] [Google Scholar]
- Bray B., Lane D. A., Freyssinet J. M., Pejler G., Lindahl U. Anti-thrombin activities of heparin. Effect of saccharide chain length on thrombin inhibition by heparin cofactor II and by antithrombin. Biochem J. 1989 Aug 15;262(1):225–232. doi: 10.1042/bj2620225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. O., Borg J. Y., George P. M., Soria C., Soria J., Caen J., Carrell R. W. New carbohydrate site in mutant antithrombin (7 Ile----Asn) with decreased heparin affinity. FEBS Lett. 1988 Sep 12;237(1-2):118–122. doi: 10.1016/0014-5793(88)80183-2. [DOI] [PubMed] [Google Scholar]
- Broze G. J., Jr, Girard T. J., Novotny W. F. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry. 1990 Aug 21;29(33):7539–7546. doi: 10.1021/bi00485a001. [DOI] [PubMed] [Google Scholar]
- Broze G. J., Jr, Warren L. A., Novotny W. F., Higuchi D. A., Girard J. J., Miletich J. P. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988 Feb;71(2):335–343. [PubMed] [Google Scholar]
- Broze G. J., Jr, Wesselschmidt R., Higuchi D., Girard T., Likert K., MacPhail L., Wun T. C. The interaction between LACI and heparin. Adv Exp Med Biol. 1992;313:189–197. doi: 10.1007/978-1-4899-2444-5_19. [DOI] [PubMed] [Google Scholar]
- Busch C., Owen W. G. Identification in vitro of an endothelial cell surface cofactor for antithrombin III. Parallel studies with isolated perfused rat hearts and microcarrier cultures of bovine endothelium. J Clin Invest. 1982 Mar;69(3):726–729. doi: 10.1172/JCI110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin S., Lindhout T., Hemker H. C. The mode of action of heparin in plasma. Thromb Haemost. 1988 Dec 22;60(3):457–462. [PubMed] [Google Scholar]
- Canfield W. M., Kisiel W. Evidence of normal functional levels of activated protein C inhibitor in combined Factor V/VIII deficiency disease. J Clin Invest. 1982 Dec;70(6):1260–1272. doi: 10.1172/JCI110725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso R., Lane D. A., Thompson E. A., Olds R. J., Thein S. L., Panico M., Blench I., Morris H. R., Freyssinet J. M., Aiach M. Antithrombin Vicenza, Ala 384 to Pro (GCA to CCA) mutation, transforming the inhibitor into a substrate. Br J Haematol. 1991 Jan;77(1):87–92. doi: 10.1111/j.1365-2141.1991.tb07953.x. [DOI] [PubMed] [Google Scholar]
- Casu B., Oreste P., Torri G., Zoppetti G., Choay J., Lormeau J. C., Petitou M., Sinäy P. The structure of heparin oligosaccharide fragments with high anti-(factor Xa) activity containing the minimal antithrombin III-binding sequence. Chemical and 13C nuclear-magnetic-resonance studies. Biochem J. 1981 Sep 1;197(3):599–609. doi: 10.1042/bj1970599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casu B., Petitou M., Provasoli M., Sinaÿ P. Conformational flexibility: a new concept for explaining binding and biological properties of iduronic acid-containing glycosaminoglycans. Trends Biochem Sci. 1988 Jun;13(6):221–225. doi: 10.1016/0968-0004(88)90088-6. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. Binding of heparin to human antithrombin III activates selective chemical modification at lysine 236. Lys-107, Lys-125, and Lys-136 are situated within the heparin-binding site of antithrombin III. J Biol Chem. 1989 Feb 25;264(6):3111–3115. [PubMed] [Google Scholar]
- Chang J. Y., Tran T. H. Antithrombin III Basel. Identification of a Pro-Leu substitution in a hereditary abnormal antithrombin with impaired heparin cofactor activity. J Biol Chem. 1986 Jan 25;261(3):1174–1176. [PubMed] [Google Scholar]
- Choay J., Petitou M., Lormeau J. C., Sinaÿ P., Casu B., Gatti G. Structure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- Church F. C., Meade J. B., Treanor R. E., Whinna H. C. Antithrombin activity of fucoidan. The interaction of fucoidan with heparin cofactor II, antithrombin III, and thrombin. J Biol Chem. 1989 Feb 25;264(6):3618–3623. [PubMed] [Google Scholar]
- Church F. C., Pratt C. W., Hoffman M. Leukocyte chemoattractant peptides from the serpin heparin cofactor II. J Biol Chem. 1991 Jan 15;266(2):704–709. [PubMed] [Google Scholar]
- Colburn P., Buonassisi V. Anti-clotting activity of endothelial cell cultures and heparan sulfate proteoglycans. Biochem Biophys Res Commun. 1982 Jan 15;104(1):220–227. doi: 10.1016/0006-291x(82)91962-3. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Wagner S. L., Farrell D. H. Regulation of protease nexin-1 activity by heparin and heparan sulfate. Adv Exp Med Biol. 1992;313:297–306. doi: 10.1007/978-1-4899-2444-5_29. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B. Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb Haemost. 1991 Jul 12;66(1):49–61. [PubMed] [Google Scholar]
- Dahlbäck B., Stenflo J. Inhibitory effect of activated protein C on activation of prothrombin by platelet-bound factor Xa. Eur J Biochem. 1980 Jun;107(2):331–335. doi: 10.1111/j.1432-1033.1980.tb06033.x. [DOI] [PubMed] [Google Scholar]
- Danielsson A., Raub E., Lindahl U., Björk I. Role of ternary complexes, in which heparin binds both antithrombin and proteinase, in the acceleration of the reactions between antithrombin and thrombin or factor Xa. J Biol Chem. 1986 Nov 25;261(33):15467–15473. [PubMed] [Google Scholar]
- DeBault L. E., Esmon N. L., Olson J. R., Esmon C. T. Distribution of the thrombomodulin antigen in the rabbit vasculature. Lab Invest. 1986 Feb;54(2):172–178. [PubMed] [Google Scholar]
- Devraj-Kizuk R., Chui D. H., Prochownik E. V., Carter C. J., Ofosu F. A., Blajchman M. A. Antithrombin-III-Hamilton: a gene with a point mutation (guanine to adenine) in codon 382 causing impaired serine protease reactivity. Blood. 1988 Nov;72(5):1518–1523. [PubMed] [Google Scholar]
- Dittman W. A., Kumada T., Sadler J. E., Majerus P. W. The structure and function of mouse thrombomodulin. Phorbol myristate acetate stimulates degradation and synthesis of thrombomodulin without affecting mRNA levels in hemangioma cells. J Biol Chem. 1988 Oct 25;263(30):15815–15822. [PubMed] [Google Scholar]
- Dittman W. A., Majerus P. W. Sequence of a cDNA for mouse thrombomodulin and comparison of the predicted mouse and human amino acid sequences. Nucleic Acids Res. 1989 Jan 25;17(2):802–802. doi: 10.1093/nar/17.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGEBERG O. INHERITED ANTITHROMBIN DEFICIENCY CAUSING THROMBOPHILIA. Thromb Diath Haemorrh. 1965 Jun 15;13:516–530. [PubMed] [Google Scholar]
- Edge A. S., Spiro R. G. Characterization of novel sequences containing 3-O-sulfated glucosamine in glomerular basement membrane heparan sulfate and localization of sulfated disaccharides to a peripheral domain. J Biol Chem. 1990 Sep 15;265(26):15874–15881. [PubMed] [Google Scholar]
- Ehrlich H. J., Keijer J., Preissner K. T., Gebbink R. K., Pannekoek H. Functional interaction of plasminogen activator inhibitor type 1 (PAI-1) and heparin. Biochemistry. 1991 Jan 29;30(4):1021–1028. doi: 10.1021/bi00218a020. [DOI] [PubMed] [Google Scholar]
- Engh R. A., Wright H. T., Huber R. Modeling the intact form of the alpha 1-proteinase inhibitor. Protein Eng. 1990 May;3(6):469–477. doi: 10.1093/protein/3.6.469. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L., Harris K. W. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982 Jul 25;257(14):7944–7947. [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989 Mar 25;264(9):4743–4746. [PubMed] [Google Scholar]
- Esmon N. L., Carroll R. C., Esmon C. T. Thrombomodulin blocks the ability of thrombin to activate platelets. J Biol Chem. 1983 Oct 25;258(20):12238–12242. [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Farrell D. H., Cunningham D. D. Glycosaminoglycans on fibroblasts accelerate thrombin inhibition by protease nexin-1. Biochem J. 1987 Jul 15;245(2):543–550. doi: 10.1042/bj2450543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell D. H., Cunningham D. D. Human fibroblasts accelerate the inhibition of thrombin by protease nexin. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6858–6862. doi: 10.1073/pnas.83.18.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell D. H., Wagner S. L., Yuan R. H., Cunningham D. D. Localization of protease nexin-1 on the fibroblast extracellular matrix. J Cell Physiol. 1988 Feb;134(2):179–188. doi: 10.1002/jcp.1041340203. [DOI] [PubMed] [Google Scholar]
- Fay W. P., Owen W. G. Platelet plasminogen activator inhibitor: purification and characterization of interaction with plasminogen activators and activated protein C. Biochemistry. 1989 Jul 11;28(14):5773–5778. doi: 10.1021/bi00440a011. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin. Ann N Y Acad Sci. 1986;485:5–15. doi: 10.1111/j.1749-6632.1986.tb34563.x. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Gailani D., Broze G. J., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991 Aug 23;253(5022):909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985 Sep 15;230(3):665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandrille S., Aiach M., Lane D. A., Vidaud D., Molho-Sabatier P., Caso R., de Moerloose P., Fiessinger J. N., Clauser E. Important role of arginine 129 in heparin-binding site of antithrombin III. Identification of a novel mutation arginine 129 to glutamine. J Biol Chem. 1990 Nov 5;265(31):18997–19001. [PubMed] [Google Scholar]
- Geiger M., Heeb M. J., Binder B. R., Griffin J. H. Competition of activated protein C and urokinase for a heparin-dependent inhibitor. FASEB J. 1988 Apr;2(7):2263–2267. doi: 10.1096/fasebj.2.7.3350241. [DOI] [PubMed] [Google Scholar]
- Gettins P., Wooten E. W. On the domain structure of antithrombin III. Tentative localization of the heparin binding region using 1H NMR spectroscopy. Biochemistry. 1987 Jul 14;26(14):4403–4408. doi: 10.1021/bi00388a032. [DOI] [PubMed] [Google Scholar]
- Girard T. J., Warren L. A., Novotny W. F., Likert K. M., Brown S. G., Miletich J. P., Broze G. J., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989 Apr 6;338(6215):518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- Gomi K., Zushi M., Honda G., Kawahara S., Matsuzaki O., Kanabayashi T., Yamamoto S., Maruyama I., Suzuki K. Antithrombotic effect of recombinant human thrombomodulin on thrombin-induced thromboembolism in mice. Blood. 1990 Apr 1;75(7):1396–1399. [PubMed] [Google Scholar]
- Griffith M. J. Heparin-catalyzed inhibitor/protease reactions: kinetic evidence for a common mechanism of action of heparin. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5460–5464. doi: 10.1073/pnas.80.18.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M. J. Kinetics of the heparin-enhanced antithrombin III/thrombin reaction. Evidence for a template model for the mechanism of action of heparin. J Biol Chem. 1982 Jul 10;257(13):7360–7365. [PubMed] [Google Scholar]
- Griffith M. J., Noyes C. M., Church F. C. Reactive site peptide structural similarity between heparin cofactor II and antithrombin III. J Biol Chem. 1985 Feb 25;260(4):2218–2225. [PubMed] [Google Scholar]
- Griffith M. J., Noyes C. M., Tyndall J. A., Church F. C. Structural evidence for leucine at the reactive site of heparin cofactor II. Biochemistry. 1985 Nov 19;24(24):6777–6782. doi: 10.1021/bi00345a008. [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Neurite outgrowth activity of protease nexin-1 on neuroblastoma cells requires thrombin inhibition. J Cell Physiol. 1990 Jan;142(1):155–162. doi: 10.1002/jcp.1041420119. [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci U S A. 1988 May;85(10):3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. R., Kimura J. H., Hascall V. C. Proteoglycan core protein families. Annu Rev Biochem. 1986;55:539–567. doi: 10.1146/annurev.bi.55.070186.002543. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Zushi M., Yamamoto S., Suzuki K. Further localization of binding sites for thrombin and protein C in human thrombomodulin. J Biol Chem. 1990 Nov 25;265(33):20156–20159. [PubMed] [Google Scholar]
- Heeb M. J., España F., Geiger M., Collen D., Stump D. C., Griffin J. H. Immunological identity of heparin-dependent plasma and urinary protein C inhibitor and plasminogen activator inhibitor-3. J Biol Chem. 1987 Nov 25;262(33):15813–15816. [PubMed] [Google Scholar]
- Heeb M. J., Griffin J. H. Physiologic inhibition of human activated protein C by alpha 1-antitrypsin. J Biol Chem. 1988 Aug 25;263(24):11613–11616. [PubMed] [Google Scholar]
- Heuck C. C., Schiele U., Horn D., Fronda D., Ritz E. The role of surface charge on the accelerating action of heparin on the antithrombin III-inhibited activity of alpha-thrombin. J Biol Chem. 1985 Apr 25;260(8):4598–4603. [PubMed] [Google Scholar]
- High K. A. Antithrombin III, protein C, and protein S. Naturally occurring anticoagulant proteins. Arch Pathol Lab Med. 1988 Jan;112(1):28–36. [PubMed] [Google Scholar]
- Hirahara K., Koyama M., Matsuishi T., Kurata M. The effect of human thrombomodulin on the inactivation of thrombin by human antithrombin III. Thromb Res. 1990 Jan 1;57(1):117–126. doi: 10.1016/0049-3848(90)90200-v. [DOI] [PubMed] [Google Scholar]
- Hofsteenge J., Stone S. R. The effect of thrombomodulin on the cleavage of fibrinogen and fibrinogen fragments by thrombin. Eur J Biochem. 1987 Oct 1;168(1):49–56. doi: 10.1111/j.1432-1033.1987.tb13385.x. [DOI] [PubMed] [Google Scholar]
- Hofsteenge J., Taguchi H., Stone S. R. Effect of thrombomodulin on the kinetics of the interaction of thrombin with substrates and inhibitors. Biochem J. 1986 Jul 1;237(1):243–251. doi: 10.1042/bj2370243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg P. J., Jackson C. M. Fibrin monomer protects thrombin from inactivation by heparin-antithrombin III: implications for heparin efficacy. Proc Natl Acad Sci U S A. 1989 May;86(10):3619–3623. doi: 10.1073/pnas.86.10.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg P. J., Jackson C. M. Formation of a ternary complex between thrombin, fibrin monomer, and heparin influences the action of thrombin on its substrates. J Biol Chem. 1990 Jan 5;265(1):248–255. [PubMed] [Google Scholar]
- Hogg P. J., Jackson C. M. Heparin promotes the binding of thrombin to fibrin polymer. Quantitative characterization of a thrombin-fibrin polymer-heparin ternary complex. J Biol Chem. 1990 Jan 5;265(1):241–247. [PubMed] [Google Scholar]
- Holmer E., Kurachi K., Söderström G. The molecular-weight dependence of the rate-enhancing effect of heparin on the inhibition of thrombin, factor Xa, factor IXa, factor XIa, factor XIIa and kallikrein by antithrombin. Biochem J. 1981 Feb 1;193(2):395–400. doi: 10.1042/bj1930395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer E., Lindahl U., Bäckström G., Thunberg L., Sandberg H., Söderström G., Anderson L. O. Anticoagulant activities and effects on platelets of a heparin fragment with high affinity for antithrombin. Thromb Res. 1980 Jun 15;18(6):861–869. doi: 10.1016/0049-3848(80)90208-x. [DOI] [PubMed] [Google Scholar]
- Holmer E., Söderström G., Andersson L. O. Studies on the mechanism of the rate-enhancing effect of heparin on the thrombin-antithrombin III reaction. Eur J Biochem. 1979 Jan 2;93(1):1–5. doi: 10.1111/j.1432-1033.1979.tb12787.x. [DOI] [PubMed] [Google Scholar]
- Horner A. A., Kusche M., Lindahl U., Peterson C. B. Determination of the range in binding-site densities of rat skin heparin chains with high binding affinities for antithrombin. Biochem J. 1988 Apr 1;251(1):141–145. doi: 10.1042/bj2510141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A. Rat heparan sulphates. A study of the antithrombin-binding properties of heparan sulphate chains from rat adipose tissue, brain, carcase, heart, intestine, kidneys, liver, lungs, skin and spleen. Biochem J. 1990 Mar 1;266(2):553–559. [PMC free article] [PubMed] [Google Scholar]
- Horner A. A. Rat heparins. A study of the relative sizes and antithrombin-binding characteristics of heparin proteoglycans, chains and depolymerization products from rat adipose tissue, heart, lungs, peritoneal cavity and skin. Biochem J. 1986 Nov 15;240(1):171–179. doi: 10.1042/bj2400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortin G. L., Benutto B. M. Inhibition of thrombin's clotting activity by synthetic peptide segments of its inhibitors and substrates. Biochem Biophys Res Commun. 1990 Jun 15;169(2):437–442. doi: 10.1016/0006-291x(90)90350-v. [DOI] [PubMed] [Google Scholar]
- Hortin G. L., Tollefsen D. M., Benutto B. M. Antithrombin activity of a peptide corresponding to residues 54-75 of heparin cofactor II. J Biol Chem. 1989 Aug 25;264(24):13979–13982. [PubMed] [Google Scholar]
- Hortin G., Tollefsen D. M., Strauss A. W. Identification of two sites of sulfation of human heparin cofactor II. J Biol Chem. 1986 Dec 5;261(34):15827–15830. [PubMed] [Google Scholar]
- Hovingh P., Piepkorn M., Linker A. Biological implications of the structural, antithrombin affinity and anticoagulant activity relationships among vertebrate heparins and heparan sulphates. Biochem J. 1986 Jul 15;237(2):573–581. doi: 10.1042/bj2370573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoylaerts M., Owen W. G., Collen D. Involvement of heparin chain length in the heparin-catalyzed inhibition of thrombin by antithrombin III. J Biol Chem. 1984 May 10;259(9):5670–5677. [PubMed] [Google Scholar]
- Huber R., Carrell R. W. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989 Nov 14;28(23):8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- Hurst R. E., Poon M. C., Griffith M. J. Structure-activity relationships of heparin. Independence of heparin charge density and antithrombin-binding domains in thrombin inhibition by antithrombin and heparin cofactor II. J Clin Invest. 1983 Sep;72(3):1042–1045. doi: 10.1172/JCI111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Iverius P. H. Distribution of sulphate and iduronic acid residues in heparin and heparan sulphate. Biochem J. 1974 Jan;137(1):33–43. doi: 10.1042/bj1370033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn R. C., Tollefsen D. M. Isolation and characterization of a partial cDNA clone for heparin cofactor II1. Biochem Biophys Res Commun. 1986 May 29;137(1):431–436. doi: 10.1016/0006-291x(86)91228-3. [DOI] [PubMed] [Google Scholar]
- Ireland H., Lane D. A., Thompson E., Walker I. D., Blench I., Morris H. R., Freyssinet J. M., Grunebaum L., Olds R., Thein S. L. Antithrombin Glasgow II: alanine 382 to threonine mutation in the serpin P12 position, resulting in a substrate reaction with thrombin. Br J Haematol. 1991 Sep;79(1):70–74. doi: 10.1111/j.1365-2141.1991.tb08009.x. [DOI] [PubMed] [Google Scholar]
- Ishii H., Salem H. H., Bell C. E., Laposata E. A., Majerus P. W. Thrombomodulin, an endothelial anticoagulant protein, is absent from the human brain. Blood. 1986 Feb;67(2):362–365. [PubMed] [Google Scholar]
- Jackman R. W., Beeler D. L., Fritze L., Soff G., Rosenberg R. D. Human thrombomodulin gene is intron depleted: nucleic acid sequences of the cDNA and gene predict protein structure and suggest sites of regulatory control. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6425–6429. doi: 10.1073/pnas.84.18.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman R. W., Beeler D. L., VanDeWater L., Rosenberg R. D. Characterization of a thrombomodulin cDNA reveals structural similarity to the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8834–8838. doi: 10.1073/pnas.83.23.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Busch S. J., Cardin A. D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991 Apr;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. V., Kline M. D., Owen W. G. The effect of bovine thrombomodulin on the specificity of bovine thrombin. J Biol Chem. 1986 Mar 15;261(8):3876–3882. [PubMed] [Google Scholar]
- Jane S. M., Mitchell C. A., Hau L., Salem H. H. Inhibition of activated protein C by platelets. J Clin Invest. 1989 Jan;83(1):222–226. doi: 10.1172/JCI113862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R. E., Oosta G. M., Gardner W. T., Rosenberg R. D. The kinetics of hemostatic enzyme-antithrombin interactions in the presence of low molecular weight heparin. J Biol Chem. 1980 Nov 10;255(21):10081–10090. [PubMed] [Google Scholar]
- Karlsson K., Lindahl U., Marklund S. L. Binding of human extracellular superoxide dismutase C to sulphated glycosaminoglycans. Biochem J. 1988 Nov 15;256(1):29–33. doi: 10.1042/bj2560029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama Y., Niwa M., Yamagishi R., Takahashi K., Sakuragawa N., Koide T. Specificity of sulfated polysaccharides to accelerate the inhibition of activated protein C by protein C inhibitor. Thromb Res. 1987 Oct 15;48(2):179–185. doi: 10.1016/0049-3848(87)90414-2. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Linhardt R. J. Structural features of heparin and their effect on heparin cofactor II mediated inhibition of thrombin. Thromb Res. 1989 Jan 1;53(1):55–71. doi: 10.1016/0049-3848(89)90115-1. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Canfield W. M., Ericsson L. H., Davie E. W. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977 Dec 27;16(26):5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- Kisiel W. Human plasma protein C: isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Invest. 1979 Sep;64(3):761–769. doi: 10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Koide T., Odani S., Takahashi K., Ono T., Sakuragawa N. Antithrombin III Toyama: replacement of arginine-47 by cysteine in hereditary abnormal antithrombin III that lacks heparin-binding ability. Proc Natl Acad Sci U S A. 1984 Jan;81(2):289–293. doi: 10.1073/pnas.81.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Leone C. W., Marchildon G. A., Marcum J. A., Rosenberg R. D. Isolation and characterization of heparan sulfate proteoglycans produced by cloned rat microvascular endothelial cells. J Biol Chem. 1992 Mar 5;267(7):4859–4869. [PubMed] [Google Scholar]
- Kojima T., Shworak N. W., Rosenberg R. D. Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate proteoglycan core proteins from a rat endothelial cell line. J Biol Chem. 1992 Mar 5;267(7):4870–4877. [PubMed] [Google Scholar]
- Koyama T., Parkinson J. F., Sié P., Bang N. U., Müller-Berghaus G., Preissner K. T. Different glycoforms of human thrombomodulin. Their glycosaminoglycan-dependent modulatory effects on thrombin inactivation by heparin cofactor II and antithrombin III. Eur J Biochem. 1991 Jun 15;198(3):563–570. doi: 10.1111/j.1432-1033.1991.tb16051.x. [DOI] [PubMed] [Google Scholar]
- Kuhn L. A., Griffin J. H., Fisher C. L., Greengard J. S., Bouma B. N., España F., Tainer J. A. Elucidating the structural chemistry of glycosaminoglycan recognition by protein C inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8506–8510. doi: 10.1073/pnas.87.21.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa S., Aoki N. Preparation of thrombomodulin from human placenta. Thromb Res. 1985 Feb 1;37(3):353–364. doi: 10.1016/0049-3848(85)90064-7. [DOI] [PubMed] [Google Scholar]
- Kurosawa S., Galvin J. B., Esmon N. L., Esmon C. T. Proteolytic formation and properties of functional domains of thrombomodulin. J Biol Chem. 1987 Feb 15;262(5):2206–2212. [PubMed] [Google Scholar]
- Kurosawa S., Stearns D. J., Jackson K. W., Esmon C. T. A 10-kDa cyanogen bromide fragment from the epidermal growth factor homology domain of rabbit thrombomodulin contains the primary thrombin binding site. J Biol Chem. 1988 May 5;263(13):5993–5996. [PubMed] [Google Scholar]
- Kusche M., Bäckström G., Riesenfeld J., Petitou M., Choay J., Lindahl U. Biosynthesis of heparin. O-sulfation of the antithrombin-binding region. J Biol Chem. 1988 Oct 25;263(30):15474–15484. [PubMed] [Google Scholar]
- Kusche M., Lindahl U. Biosynthesis of heparin. O-sulfation of D-glucuronic acid units. J Biol Chem. 1990 Sep 15;265(26):15403–15409. [PubMed] [Google Scholar]
- Kusche M., Torri G., Casu B., Lindahl U. Biosynthesis of heparin. Availability of glucosaminyl 3-O-sulfation sites. J Biol Chem. 1990 May 5;265(13):7292–7300. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Lane D. A., Denton J., Flynn A. M., Thunberg L., Lindahl U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem J. 1984 Mar 15;218(3):725–732. doi: 10.1042/bj2180725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. A., Flynn A. M., Pejler G., Lindahl U., Choay J., Preissner K. Structural requirements for the neutralization of heparin-like saccharides by complement S protein/vitronectin. J Biol Chem. 1987 Dec 5;262(34):16343–16348. [PubMed] [Google Scholar]
- Lane D. A., Pejler G., Flynn A. M., Thompson E. A., Lindahl U. Neutralization of heparin-related saccharides by histidine-rich glycoprotein and platelet factor 4. J Biol Chem. 1986 Mar 25;261(9):3980–3986. [PubMed] [Google Scholar]
- Laug W. E., Aebersold R., Jong A., Rideout W., Bergman B. L., Baker J. Isolation of multiple types of plasminogen activator inhibitors from vascular smooth muscle cells. Thromb Haemost. 1989 Jun 30;61(3):517–521. [PubMed] [Google Scholar]
- Laurell M., Stenflo J. Protein C inhibitor from human plasma: characterization of native and cleaved inhibitor and demonstration of inhibitor complexes with plasma kallikrein. Thromb Haemost. 1989 Nov 24;62(3):885–891. [PubMed] [Google Scholar]
- Laurent T. C., Tengblad A., Thunberg L., Hök M., Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978 Nov 1;175(2):691–701. doi: 10.1042/bj1750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Thunberg L., Leder I. G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Thunberg L. The antithrombin-binding sequence in heparin. Identification of an essential 6-O-sulfate group. J Biol Chem. 1983 Aug 25;258(16):9826–9830. [PubMed] [Google Scholar]
- Lindahl U., Kjellén L. Heparin or heparan sulfate--what is the difference? Thromb Haemost. 1991 Jul 12;66(1):44–48. [PubMed] [Google Scholar]
- Lindahl U., Pejler G., Bøgwald J., Seljelid R. A prothrombinase complex of mouse peritoneal macrophages. Arch Biochem Biophys. 1989 Aug 15;273(1):180–188. doi: 10.1016/0003-9861(89)90177-x. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Thunberg L., Bäckström G., Riesenfeld J., Nordling K., Björk I. Extension and structural variability of the antithrombin-binding sequence in heparin. J Biol Chem. 1984 Oct 25;259(20):12368–12376. [PubMed] [Google Scholar]
- Lindblom A., Bengtsson-Olivecrona G., Fransson L. A. Domain structure of endothelial heparan sulphate. Biochem J. 1991 Nov 1;279(Pt 3):821–829. doi: 10.1042/bj2790821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollar P., MacIntosh S. C., Owen W. G. Reaction of antithrombin III with thrombin bound to the vascular endothelium. Analysis in a recirculating perfused rabbit heart preparation. J Biol Chem. 1984 Apr 10;259(7):4335–4338. [PubMed] [Google Scholar]
- Lollar P., Owen W. G. Clearance of thrombin from circulation in rabbits by high-affinity binding sites on endothelium. Possible role in the inactivation of thrombin by antithrombin III. J Clin Invest. 1980 Dec;66(6):1222–1230. doi: 10.1172/JCI109973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R. L., Esmon N. L., Esmon C. T., Johnson A. E. The active site of the thrombin-thrombomodulin complex. A fluorescence energy transfer measurement of its distance above the membrane surface. J Biol Chem. 1989 Aug 5;264(22):12956–12962. [PubMed] [Google Scholar]
- Machovich R., Arányi P. Effect of heparin on thrombin inactivation by antithrombin-III. Biochem J. 1978 Sep 1;173(3):869–875. doi: 10.1042/bj1730869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machovich R., Staub M., Patthy L. Decreased heparin sensitivity of cycholhexanedione-modified thrombin. Eur J Biochem. 1978 Feb;83(2):473–477. doi: 10.1111/j.1432-1033.1978.tb12113.x. [DOI] [PubMed] [Google Scholar]
- Maimone M. M., Tollefsen D. M. Activation of heparin cofactor II by heparin oligosaccharides. Biochem Biophys Res Commun. 1988 May 16;152(3):1056–1061. doi: 10.1016/s0006-291x(88)80391-7. [DOI] [PubMed] [Google Scholar]
- Maimone M. M., Tollefsen D. M. Structure of a dermatan sulfate hexasaccharide that binds to heparin cofactor II with high affinity. J Biol Chem. 1990 Oct 25;265(30):18263–18271. [PubMed] [Google Scholar]
- Marcum J. A., Atha D. H., Fritze L. M., Nawroth P., Stern D., Rosenberg R. D. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986 Jun 5;261(16):7507–7517. [PubMed] [Google Scholar]
- Marcum J. A., McKenney J. B., Rosenberg R. D. Acceleration of thrombin-antithrombin complex formation in rat hindquarters via heparinlike molecules bound to the endothelium. J Clin Invest. 1984 Aug;74(2):341–350. doi: 10.1172/JCI111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- Marlar R. A., Griffin J. H. Deficiency of protein C inhibitor in combined factor V/VIII deficiency disease. J Clin Invest. 1980 Nov;66(5):1186–1189. doi: 10.1172/JCI109952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlar R. A., Kleiss A. J., Griffin J. H. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood. 1982 May;59(5):1067–1072. [PubMed] [Google Scholar]
- Maruyama I., Bell C. E., Majerus P. W. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985 Aug;101(2):363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama I., Salem H. H., Ishii H., Majerus P. W. Human thrombomodulin is not an efficient inhibitor of the procoagulant activity of thrombin. J Clin Invest. 1985 Mar;75(3):987–991. doi: 10.1172/JCI111800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers J. C., Vlooswijk R. A., Kanters D. H., Hessing M., Bouma B. N. Identification of monoclonal antibodies that inhibit the function of protein C inhibitor. Evidence for heparin-independent inhibition of activated protein C in plasma. Blood. 1988 Oct;72(4):1401–1403. [PubMed] [Google Scholar]
- Molho-Sabatier P., Aiach M., Gaillard I., Fiessinger J. N., Fischer A. M., Chadeuf G., Clauser E. Molecular characterization of antithrombin III (ATIII) variants using polymerase chain reaction. Identification of the ATIII Charleville as an Ala 384 Pro mutation. J Clin Invest. 1989 Oct;84(4):1236–1242. doi: 10.1172/JCI114290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottonen J., Strand A., Symersky J., Sweet R. M., Danley D. E., Geoghegan K. F., Gerard R. D., Goldsmith E. J. Structural basis of latency in plasminogen activator inhibitor-1. Nature. 1992 Jan 16;355(6357):270–273. doi: 10.1038/355270a0. [DOI] [PubMed] [Google Scholar]
- Mourey L., Samama J. P., Delarue M., Choay J., Lormeau J. C., Petitou M., Moras D. Antithrombin III: structural and functional aspects. Biochimie. 1990 Aug;72(8):599–608. doi: 10.1016/0300-9084(90)90123-x. [DOI] [PubMed] [Google Scholar]
- Munakata H., Hsu C. C., Kodama C., Aikawa J., Sakurada M., Ototani N., Isemura M., Yosizawa Z., Hayashi N. Isolation of dermatan sulfate with high heparin cofactor II-mediated thrombin-inhibitory activity from porcine spleen. Biochim Biophys Acta. 1987 Sep 11;925(3):325–331. doi: 10.1016/0304-4165(87)90198-x. [DOI] [PubMed] [Google Scholar]
- Murata M., Ikeda Y., Araki Y., Murakami H., Sato K., Yamamoto M., Watanabe K., Ando Y., Igawa T., Maruyama I. Inhibition by endothelial cells of platelet aggregating activity of thrombin--role of thrombomodulin. Thromb Res. 1988 Jun 1;50(5):647–656. doi: 10.1016/0049-3848(88)90323-4. [DOI] [PubMed] [Google Scholar]
- Musci G., Berliner L. J., Esmon C. T. Evidence for multiple conformational changes in the active center of thrombin induced by complex formation with thrombomodulin: an analysis employing nitroxide spin-labels. Biochemistry. 1988 Jan 26;27(2):769–773. doi: 10.1021/bi00402a042. [DOI] [PubMed] [Google Scholar]
- Nawa K., Sakano K., Fujiwara H., Sato Y., Sugiyama N., Teruuchi T., Iwamoto M., Marumoto Y. Presence and function of chondroitin-4-sulfate on recombinant human soluble thrombomodulin. Biochem Biophys Res Commun. 1990 Sep 14;171(2):729–737. doi: 10.1016/0006-291x(90)91207-9. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E. A simple rate law that describes the kinetics of the heparin-catalyzed reaction between antithrombin III and thrombin. J Biol Chem. 1983 Dec 10;258(23):14708–14717. [PubMed] [Google Scholar]
- Ofosu F. A., Blajchman M. A., Modi G. J., Smith L. M., Buchanan M. R., Hirsh J. The importance of thrombin inhibition for the expression of the anticoagulant activities of heparin, dermatan sulphate, low molecular weight heparin and pentosan polysulphate. Br J Haematol. 1985 Aug;60(4):695–704. doi: 10.1111/j.1365-2141.1985.tb07474.x. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Choay J., Anvari N., Smith L. M., Blajchman M. A. Inhibition of factor X and factor V activation by dermatan sulfate and a pentasaccharide with high affinity for antithrombin III in human plasma. Eur J Biochem. 1990 Oct 24;193(2):485–493. doi: 10.1111/j.1432-1033.1990.tb19363.x. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Hirsh J., Esmon C. T., Modi G. J., Smith L. M., Anvari N., Buchanan M. R., Fenton J. W., 2nd, Blajchman M. A. Unfractionated heparin inhibits thrombin-catalysed amplification reactions of coagulation more efficiently than those catalysed by factor Xa. Biochem J. 1989 Jan 1;257(1):143–150. doi: 10.1042/bj2570143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofosu F. A., Sie P., Modi G. J., Fernandez F., Buchanan M. R., Blajchman M. A., Boneu B., Hirsh J. The inhibition of thrombin-dependent positive-feedback reactions is critical to the expression of the anticoagulant effect of heparin. Biochem J. 1987 Apr 15;243(2):579–588. doi: 10.1042/bj2430579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwusidi J. I., Anvari N., Kulczycky M., Blajchman M. A., Buchanan M. R., Ofosu F. A. Fibrin moderates the catalytic action of heparin but not that of dermatan sulfate on thrombin inhibition in human plasma. J Lab Clin Med. 1991 May;117(5):359–364. [PubMed] [Google Scholar]
- Olds R. J., Lane D. A., Boisclair M., Sas G., Bock S. C., Thein S. L. Antithrombin Budapest 3. An antithrombin variant with reduced heparin affinity resulting from the substitution L99F. FEBS Lett. 1992 Apr 6;300(3):241–246. doi: 10.1016/0014-5793(92)80854-a. [DOI] [PubMed] [Google Scholar]
- Olson S. T., Björk I. Predominant contribution of surface approximation to the mechanism of heparin acceleration of the antithrombin-thrombin reaction. Elucidation from salt concentration effects. J Biol Chem. 1991 Apr 5;266(10):6353–6364. [PubMed] [Google Scholar]
- Olson S. T., Halvorson H. R., Björk I. Quantitative characterization of the thrombin-heparin interaction. Discrimination between specific and nonspecific binding models. J Biol Chem. 1991 Apr 5;266(10):6342–6352. [PubMed] [Google Scholar]
- Olson S. T. Heparin and ionic strength-dependent conversion of antithrombin III from an inhibitor to a substrate of alpha-thrombin. J Biol Chem. 1985 Aug 25;260(18):10153–10160. [PubMed] [Google Scholar]
- Olson S. T., Shore J. D. Transient kinetics of heparin-catalyzed protease inactivation by antithrombin III. The reaction step limiting heparin turnover in thrombin neutralization. J Biol Chem. 1986 Oct 5;261(28):13151–13159. [PubMed] [Google Scholar]
- Olson S. T., Srinivasan K. R., Björk I., Shore J. D. Binding of high affinity heparin to antithrombin III. Stopped flow kinetic studies of the binding interaction. J Biol Chem. 1981 Nov 10;256(21):11073–11079. [PubMed] [Google Scholar]
- Olson S. T. Transient kinetics of heparin-catalyzed protease inactivation by antithrombin III. Linkage of protease-inhibitor-heparin interactions in the reaction with thrombin. J Biol Chem. 1988 Feb 5;263(4):1698–1708. [PubMed] [Google Scholar]
- Oosta G. M., Gardner W. T., Beeler D. L., Rosenberg R. D. Multiple functional domains of the heparin molecule. Proc Natl Acad Sci U S A. 1981 Feb;78(2):829–833. doi: 10.1073/pnas.78.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T. Functional properties of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1981 Jun 10;256(11):5532–5535. [PubMed] [Google Scholar]
- Parker K. A., Tollefsen D. M. The protease specificity of heparin cofactor II. Inhibition of thrombin generated during coagulation. J Biol Chem. 1985 Mar 25;260(6):3501–3505. [PubMed] [Google Scholar]
- Parkinson J. F., Garcia J. G., Bang N. U. Decreased thrombin affinity of cell-surface thrombomodulin following treatment of cultured endothelial cells with beta-D-xyloside. Biochem Biophys Res Commun. 1990 May 31;169(1):177–183. doi: 10.1016/0006-291x(90)91451-w. [DOI] [PubMed] [Google Scholar]
- Parkinson J. F., Grinnell B. W., Moore R. E., Hoskins J., Vlahos C. J., Bang N. U. Stable expression of a secretable deletion mutant of recombinant human thrombomodulin in mammalian cells. J Biol Chem. 1990 Jul 25;265(21):12602–12610. [PubMed] [Google Scholar]
- Parkinson J. F., Vlahos C. J., Yan S. C., Bang N. U. Recombinant human thrombomodulin. Regulation of cofactor activity and anticoagulant function by a glycosaminoglycan side chain. Biochem J. 1992 Apr 1;283(Pt 1):151–157. doi: 10.1042/bj2830151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaquet C., Thomas J. C., Caron L., Hauswirth N., Puel F., Berkaloff C. Light-harvesting complexes of brown algae. Biochemical characterization and immunological relationships. FEBS Lett. 1991 Mar 11;280(1):21–26. doi: 10.1016/0014-5793(91)80195-9. [DOI] [PubMed] [Google Scholar]
- Patthy L. Detecting distant homologies of mosaic proteins. Analysis of the sequences of thrombomodulin, thrombospondin complement components C9, C8 alpha and C8 beta, vitronectin and plasma cell membrane glycoprotein PC-1. J Mol Biol. 1988 Aug 20;202(4):689–696. doi: 10.1016/0022-2836(88)90550-5. [DOI] [PubMed] [Google Scholar]
- Pejler G., Bäckström G., Lindahl U., Paulsson M., Dziadek M., Fujiwara S., Timpl R. Structure and affinity for antithrombin of heparan sulfate chains derived from basement membrane proteoglycans. J Biol Chem. 1987 Apr 15;262(11):5036–5043. [PubMed] [Google Scholar]
- Pejler G., Danielsson A., Björk I., Lindahl U., Nader H. B., Dietrich C. P. Structure and antithrombin-binding properties of heparin isolated from the clams Anomalocardia brasiliana and Tivela mactroides. J Biol Chem. 1987 Aug 25;262(24):11413–11421. [PubMed] [Google Scholar]
- Pejler G., David G. Basement-membrane heparan sulphate with high affinity for antithrombin synthesized by normal and transformed mouse mammary epithelial cells. Biochem J. 1987 Nov 15;248(1):69–77. doi: 10.1042/bj2480069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. B., Blackburn M. N. Antithrombin conformation and the catalytic role of heparin. I. Does cleavage by thrombin induce structural changes in the heparin-binding region of antithrombin? J Biol Chem. 1987 Jun 5;262(16):7552–7558. [PubMed] [Google Scholar]
- Peterson C. B., Blackburn M. N. Antithrombin conformation and the catalytic role of heparin. II. Is the heparin-induced conformational change in antithrombin required for rapid inactivation of thrombin? J Biol Chem. 1987 Jun 5;262(16):7559–7566. [PubMed] [Google Scholar]
- Petitou M., Duchaussoy P., Lederman I., Choay J., Sinaÿ P. Binding of heparin to antithrombin III: a chemical proof of the critical role played by a 3-sulfated 2-amino-2-deoxy-D-glucose residue. Carbohydr Res. 1988 Aug 15;179:163–172. doi: 10.1016/0008-6215(88)84116-8. [DOI] [PubMed] [Google Scholar]
- Petitou M., Lormeau J. C., Choay J. Interaction of heparin and antithrombin III. The role of O-sulfate groups. Eur J Biochem. 1988 Oct 1;176(3):637–640. doi: 10.1111/j.1432-1033.1988.tb14324.x. [DOI] [PubMed] [Google Scholar]
- Petitou M., Lormeau J. C., Perly B., Berthault P., Bossennec V., Sié P., Choay J. Is there a unique sequence in heparin for interaction with heparin cofactor II? Structural and biological studies of heparin-derived oligosaccharides. J Biol Chem. 1988 Jun 25;263(18):8685–8690. [PubMed] [Google Scholar]
- Pomerantz M. W., Owen W. G. A catalytic role for heparin. Evidence for a ternary complex of heparin cofactor thrombin and heparin. Biochim Biophys Acta. 1978 Jul 21;535(1):66–77. doi: 10.1016/0005-2795(78)90033-8. [DOI] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C. W., Church F. C. Heparin binding to protein C inhibitor. J Biol Chem. 1992 May 5;267(13):8789–8794. [PubMed] [Google Scholar]
- Pratt C. W., Tobin R. B., Church F. C. Interaction of heparin cofactor II with neutrophil elastase and cathepsin G. J Biol Chem. 1990 Apr 15;265(11):6092–6097. [PubMed] [Google Scholar]
- Preissner K. T., Delvos U., Müller-Berghaus G. Binding of thrombin to thrombomodulin accelerates inhibition of the enzyme by antithrombin III. Evidence for a heparin-independent mechanism. Biochemistry. 1987 May 5;26(9):2521–2528. doi: 10.1021/bi00383a018. [DOI] [PubMed] [Google Scholar]
- Preissner K. T., Jenne D. Structure of vitronectin and its biological role in haemostasis. Thromb Haemost. 1991 Jul 12;66(1):123–132. [PubMed] [Google Scholar]
- Preissner K. T., Koyama T., Müller D., Tschopp J., Müller-Berghaus G. Domain structure of the endothelial cell receptor thrombomodulin as deduced from modulation of its anticoagulant functions. Evidence for a glycosaminoglycan-dependent secondary binding site for thrombin. J Biol Chem. 1990 Mar 25;265(9):4915–4922. [PubMed] [Google Scholar]
- Ragg H. A new member of the plasma protease inhibitor gene family. Nucleic Acids Res. 1986 Jan 24;14(2):1073–1088. doi: 10.1093/nar/14.2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragg H., Preibisch G. Structure and expression of the gene coding for the human serpin hLS2. J Biol Chem. 1988 Aug 25;263(24):12129–12134. [PubMed] [Google Scholar]
- Ragg H., Ulshöfer T., Gerewitz J. Glycosaminoglycan-mediated leuserpin-2/thrombin interaction. Structure-function relationships. J Biol Chem. 1990 Dec 25;265(36):22386–22391. [PubMed] [Google Scholar]
- Ragg H., Ulshöfer T., Gerewitz J. On the activation of human leuserpin-2, a thrombin inhibitor, by glycosaminoglycans. J Biol Chem. 1990 Mar 25;265(9):5211–5218. [PubMed] [Google Scholar]
- Riesenfeld J., Thunberg L., Hök M., Lindahl U. The antithrombin-binding sequence of heparin. Location of essential N-sulfate groups. J Biol Chem. 1981 Mar 10;256(5):2389–2394. [PubMed] [Google Scholar]
- Rodén L., Koerner T., Olson C., Schwartz N. B. Mechanisms of chain initiation in the biosynthesis of connective tissue polysaccharides. Fed Proc. 1985 Feb;44(2):373–380. [PubMed] [Google Scholar]
- Rosenberg R. D. Chemistry of the hemostatic mechanism and its relationship to the action of heparin. Fed Proc. 1977 Jan;36(1):10–18. [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Rosenberg R. D., de Agostini A. I. New approaches for defining sequence specific synthesis of heparan sulfate chains. Adv Exp Med Biol. 1992;313:307–316. doi: 10.1007/978-1-4899-2444-5_30. [DOI] [PubMed] [Google Scholar]
- Rosenblatt D. E., Cotman C. W., Nieto-Sampedro M., Rowe J. W., Knauer D. J. Identification of a protease inhibitor produced by astrocytes that is structurally and functionally homologous to human protease nexin-I. Brain Res. 1987 Jul 7;415(1):40–48. doi: 10.1016/0006-8993(87)90267-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Rydel T. J., Ravichandran K. G., Tulinsky A., Bode W., Huber R., Roitsch C., Fenton J. W., 2nd The structure of a complex of recombinant hirudin and human alpha-thrombin. Science. 1990 Jul 20;249(4966):277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- Sakata Y., Curriden S., Lawrence D., Griffin J. H., Loskutoff D. J. Activated protein C stimulates the fibrinolytic activity of cultured endothelial cells and decreases antiactivator activity. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1121–1125. doi: 10.1073/pnas.82.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H. H., Maruyama I., Ishii H., Majerus P. W. Isolation and characterization of thrombomodulin from human placenta. J Biol Chem. 1984 Oct 10;259(19):12246–12251. [PubMed] [Google Scholar]
- Sandset P. M., Abildgaard U., Larsen M. L. Heparin induces release of extrinsic coagulation pathway inhibitor (EPI). Thromb Res. 1988 Jun 15;50(6):803–813. doi: 10.1016/0049-3848(88)90340-4. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Scully M. F., Ellis V., Seno N., Kakkar V. V. Effect of oversulphated chondroitin and dermatan sulphate upon thrombin and factor Xa inactivation by antithrombin III or heparin cofactor II. Biochem J. 1988 Sep 1;254(2):547–551. doi: 10.1042/bj2540547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully M. F., Ellis V., Seno N., Kakkar V. V. The anticoagulant properties of mast cell product, chondroitin sulphate E. Biochem Biophys Res Commun. 1986 May 29;137(1):15–22. doi: 10.1016/0006-291x(86)91169-1. [DOI] [PubMed] [Google Scholar]
- Seldin D. C., Seno N., Austen K. F., Stevens R. L. Analysis of polysulfated chondroitin disaccharides by high-performance liquid chromatography. Anal Biochem. 1984 Aug 15;141(1):291–300. doi: 10.1016/0003-2697(84)90459-7. [DOI] [PubMed] [Google Scholar]
- Shah N., Scully M. F., Ellis V., Kakkar V. V. Influence of chemical modification of tryptophan residues on the properties of human antithrombin III. Thromb Res. 1990 Feb 1;57(3):343–352. doi: 10.1016/0049-3848(90)90250-g. [DOI] [PubMed] [Google Scholar]
- Shore J. D., Olson S. T., Craig P. A., Choay J., Björk I. Kinetics of heparin action. Ann N Y Acad Sci. 1989;556:75–80. doi: 10.1111/j.1749-6632.1989.tb22491.x. [DOI] [PubMed] [Google Scholar]
- Sie P., Dupouy D., Pichon J., Boneu B. Constitutional heparin co-factor II deficiency associated with recurrent thrombosis. Lancet. 1985 Aug 24;2(8452):414–416. doi: 10.1016/s0140-6736(85)92737-0. [DOI] [PubMed] [Google Scholar]
- Sié P., Petitou M., Lormeau J. C., Dupouy D., Boneu B., Choay J. Studies on the structural requirements of heparin for the catalysis of thrombin inhibition by heparin cofactor II. Biochim Biophys Acta. 1988 Aug 11;966(2):188–195. doi: 10.1016/0304-4165(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Skriver K., Wikoff W. R., Patston P. A., Tausk F., Schapira M., Kaplan A. P., Bock S. C. Substrate properties of C1 inhibitor Ma (alanine 434----glutamic acid). Genetic and structural evidence suggesting that the P12-region contains critical determinants of serine protease inhibitor/substrate status. J Biol Chem. 1991 May 15;266(14):9216–9221. [PubMed] [Google Scholar]
- Smith J. W., Dey N., Knauer D. J. Heparin binding domain of antithrombin III: characterization using a synthetic peptide directed polyclonal antibody. Biochemistry. 1990 Sep 25;29(38):8950–8957. doi: 10.1021/bi00490a010. [DOI] [PubMed] [Google Scholar]
- Soff G. A., Jackman R. W., Rosenberg R. D. Expression of thrombomodulin by smooth muscle cells in culture: different effects of tumor necrosis factor and cyclic adenosine monophosphate on thrombomodulin expression by endothelial cells and smooth muscle cells in culture. Blood. 1991 Feb 1;77(3):515–518. [PubMed] [Google Scholar]
- Stearns D. J., Kurosawa S., Esmon C. T. Microthrombomodulin. Residues 310-486 from the epidermal growth factor precursor homology domain of thrombomodulin will accelerate protein C activation. J Biol Chem. 1989 Feb 25;264(6):3352–3356. [PubMed] [Google Scholar]
- Stein P. E., Leslie A. G., Finch J. T., Turnell W. G., McLaughlin P. J., Carrell R. W. Crystal structure of ovalbumin as a model for the reactive centre of serpins. Nature. 1990 Sep 6;347(6288):99–102. doi: 10.1038/347099a0. [DOI] [PubMed] [Google Scholar]
- Stenflo J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976 Jan 25;251(2):355–363. [PubMed] [Google Scholar]
- Stenflo J., Fernlund P. Amino acid sequence of the heavy chain of bovine protein C. J Biol Chem. 1982 Oct 25;257(20):12180–12190. [PubMed] [Google Scholar]
- Stern D., Nawroth P., Marcum J., Handley D., Kisiel W., Rosenberg R., Stern K. Interaction of antithrombin III with bovine aortic segments. Role of heparin in binding and enhanced anticoagulant activity. J Clin Invest. 1985 Jan;75(1):272–279. doi: 10.1172/JCI111685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump D. C., Thienpont M., Collen D. Purification and characterization of a novel inhibitor of urokinase from human urine. Quantitation and preliminary characterization in plasma. J Biol Chem. 1986 Sep 25;261(27):12759–12766. [PubMed] [Google Scholar]
- Sun X. J., Chang J. Y. Evidence that arginine-129 and arginine-145 are located within the heparin binding site of human antithrombin III. Biochemistry. 1990 Sep 25;29(38):8957–8962. doi: 10.1021/bi00490a011. [DOI] [PubMed] [Google Scholar]
- Sun X. J., Chang J. Y. Heparin binding domain of human antithrombin III inferred from the sequential reduction of its three disulfide linkages. An efficient method for structural analysis of partially reduced proteins. J Biol Chem. 1989 Jul 5;264(19):11288–11293. [PubMed] [Google Scholar]
- Suzuki K., Deyashiki Y., Nishioka J., Kurachi K., Akira M., Yamamoto S., Hashimoto S. Characterization of a cDNA for human protein C inhibitor. A new member of the plasma serine protease inhibitor superfamily. J Biol Chem. 1987 Jan 15;262(2):611–616. [PubMed] [Google Scholar]
- Suzuki K., Hayashi T., Nishioka J., Kosaka Y., Zushi M., Honda G., Yamamoto S. A domain composed of epidermal growth factor-like structures of human thrombomodulin is essential for thrombin binding and for protein C activation. J Biol Chem. 1989 Mar 25;264(9):4872–4876. [PubMed] [Google Scholar]
- Suzuki K., Kusumoto H., Deyashiki Y., Nishioka J., Maruyama I., Zushi M., Kawahara S., Honda G., Yamamoto S., Horiguchi S. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987 Jul;6(7):1891–1897. doi: 10.1002/j.1460-2075.1987.tb02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kusumoto H., Hashimoto S. Isolation and characterization of thrombomodulin from bovine lung. Biochim Biophys Acta. 1986 Jul 16;882(3):343–352. doi: 10.1016/0304-4165(86)90257-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Nishioka J., Hashimoto S. Protein C inhibitor. Purification from human plasma and characterization. J Biol Chem. 1983 Jan 10;258(1):163–168. [PubMed] [Google Scholar]
- Suzuki K., Nishioka J., Hayashi T., Kosaka Y. Functionally active thrombomodulin is present in human platelets. J Biochem. 1988 Oct;104(4):628–632. doi: 10.1093/oxfordjournals.jbchem.a122523. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Nishioka J., Kusumoto H., Hashimoto S. Mechanism of inhibition of activated protein C by protein C inhibitor. J Biochem. 1984 Jan;95(1):187–195. doi: 10.1093/oxfordjournals.jbchem.a134583. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982 Mar 1;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M. Laboratory diagnosis of antithrombin and heparin cofactor II deficiency. Semin Thromb Hemost. 1990 Apr;16(2):162–168. doi: 10.1055/s-2007-1002664. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus D. W., Blank M. K. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982 Mar 10;257(5):2162–2169. [PubMed] [Google Scholar]
- Tollefsen D. M., Peacock M. E., Monafo W. J. Molecular size of dermatan sulfate oligosaccharides required to bind and activate heparin cofactor II. J Biol Chem. 1986 Jul 5;261(19):8854–8858. [PubMed] [Google Scholar]
- Tollefsen D. M., Pestka C. A., Monafo W. J. Activation of heparin cofactor II by dermatan sulfate. J Biol Chem. 1983 Jun 10;258(11):6713–6716. [PubMed] [Google Scholar]
- Tollefsen D. M., Sugimori T., Maimone M. M. Effect of low molecular weight heparin preparations on the inhibition of thrombin by heparin cofactor II. Semin Thromb Hemost. 1990 Oct;16 (Suppl):66–70. [PubMed] [Google Scholar]
- Toulon P., Moulonguet-Doleris L., Costa J. M., Aiach M. Heparin cofactor II deficiency in renal allograft recipients: no correlation with the development of thrombosis. Thromb Haemost. 1991 Jan 23;65(1):20–24. [PubMed] [Google Scholar]
- Tran T. H., Marbet G. A., Duckert F. Association of hereditary heparin co-factor II deficiency with thrombosis. Lancet. 1985 Aug 24;2(8452):413–414. doi: 10.1016/s0140-6736(85)92736-9. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Van Deerlin V. M., Tollefsen D. M. The N-terminal acidic domain of heparin cofactor II mediates the inhibition of alpha-thrombin in the presence of glycosaminoglycans. J Biol Chem. 1991 Oct 25;266(30):20223–20231. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wagner S. L., Lau A. L., Cunningham D. D. Binding of protease nexin-1 to the fibroblast surface alters its target proteinase specificity. J Biol Chem. 1989 Jan 5;264(1):611–615. [PubMed] [Google Scholar]
- Wagner S. L., Lau A. L., Nguyen A., Mimuro J., Loskutoff D. J., Isackson P. J., Cunningham D. D. Inhibitors of urokinase and thrombin in cultured neural cells. J Neurochem. 1991 Jan;56(1):234–242. doi: 10.1111/j.1471-4159.1991.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Walker F. J., Chavin S. I., Fay P. J. Inactivation of factor VIII by activated protein C and protein S. Arch Biochem Biophys. 1987 Jan;252(1):322–328. doi: 10.1016/0003-9861(87)90037-3. [DOI] [PubMed] [Google Scholar]
- Walker F. J. Regulation of activated protein C by a new protein. A possible function for bovine protein S. J Biol Chem. 1980 Jun 25;255(12):5521–5524. [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]
- Weisdorf D. J., Edson J. R. Recurrent venous thrombosis associated with inherited deficiency of heparin cofactor II. Br J Haematol. 1991 Jan;77(1):125–126. doi: 10.1111/j.1365-2141.1991.tb07961.x. [DOI] [PubMed] [Google Scholar]
- Wen D. Z., Dittman W. A., Ye R. D., Deaven L. L., Majerus P. W., Sadler J. E. Human thrombomodulin: complete cDNA sequence and chromosome localization of the gene. Biochemistry. 1987 Jul 14;26(14):4350–4357. doi: 10.1021/bi00388a025. [DOI] [PubMed] [Google Scholar]
- Whinna H. C., Blinder M. A., Szewczyk M., Tollefsen D. M., Church F. C. Role of lysine 173 in heparin binding to heparin cofactor II. J Biol Chem. 1991 May 5;266(13):8129–8135. [PubMed] [Google Scholar]
- Winnard P. T., Esmon C. T., Laue T. M. The molecular weight and oligomerization of rabbit thrombomodulin as assessed by sedimentation equilibrium. Arch Biochem Biophys. 1989 Feb 15;269(1):339–344. doi: 10.1016/0003-9861(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Wu Q. Y., Sheehan J. P., Tsiang M., Lentz S. R., Birktoft J. J., Sadler J. E. Single amino acid substitutions dissociate fibrinogen-clotting and thrombomodulin-binding activities of human thrombin. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6775–6779. doi: 10.1073/pnas.88.15.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun T. C., Kretzmer K. K., Girard T. J., Miletich J. P., Broze G. J., Jr Cloning and characterization of a cDNA coding for the lipoprotein-associated coagulation inhibitor shows that it consists of three tandem Kunitz-type inhibitory domains. J Biol Chem. 1988 May 5;263(13):6001–6004. [PubMed] [Google Scholar]
- Zushi M., Gomi K., Yamamoto S., Maruyama I., Hayashi T., Suzuki K. The last three consecutive epidermal growth factor-like structures of human thrombomodulin comprise the minimum functional domain for protein C-activating cofactor activity and anticoagulant activity. J Biol Chem. 1989 Jun 25;264(18):10351–10353. [PubMed] [Google Scholar]
- de Agostini A. I., Watkins S. C., Slayter H. S., Youssoufian H., Rosenberg R. D. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. J Cell Biol. 1990 Sep;111(3):1293–1304. doi: 10.1083/jcb.111.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]