Abstract
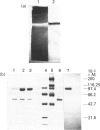
1. A soluble reduced Methyl Viologen-dependent assimilatory nitrate reductase from Azotobacter vinelandii strain UW136 grown aerobically on nitrate was purified to homogeneity by the criteria of nitrate reductase activity staining, and coincidence of a Coomassie Blue-staining protein band on polyacrylamide gels run under non-denaturing conditions. The specific activity was 3 mumol of NO2- formed/min per mg of protein. 2. Gel filtration on Superose-12 and SDS/PAGE showed that the enzyme had an M(r) of 105,000 and was monomeric. The enzyme contained 1 Mo atom, 4 Fe atoms and 4 acid-labile sulphide atoms per molecule; no evidence for the presence of cytochrome or FAD was found. 3. Mo was present in a molybdenum cofactor, which on extraction was capable of activating apo-(nit-1) nitrate reductase present in crude extracts of nit-1 mutants of Neurospora crassa. 4. As isolated, the enzyme had e.p.r. signals assigned to Mo(V) with g-values g1 = 2.023; g2 = 1.998; g3 = 1.993 and with gav. = 2.004 indicating an unusual environment of Mo in this enzyme. 5. Reduction with S2O4(2-) bleached the e.p.r. signals which, on reoxidation after the addition of NO3(2-) to initiate enzyme turnover, exhibited at short times Mo(V) signals similar to those of dissimilatory nitrate reductases, with g1 = 1.998; g2 = 1.989; g3 = 1.981 and gav. = 1.989. Prolonged incubation subsequently gave a mixture of both e.p.r. species. 6. Neither NADH nor NADPH was effective as an electron donor, but reduced Methyl Viologen (apparent Km 998 microM) and reduced Bromophenol Blue (apparent Km 158 microM) were effective. With these donors the apparent Km values for nitrate were 70 microM and 217 microM respectively.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Lactic dehydrogenase and cytochrome b2 of baker's yeast. Enzymic and chemical properties of the crystalline enzyme. Biochem J. 1959 Nov;73:539–550. doi: 10.1042/bj0730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alef K., Klemme J. H. Assimilatory nitrate reductase of Rhodopseudomonas capsulata AD2: a molybdo-hemeprotein. Z Naturforsch C. 1979 Jan-Feb;34(1-2):33–37. doi: 10.1515/znc-1979-1-210. [DOI] [PubMed] [Google Scholar]
- Bray R. C. The inorganic biochemistry of molybdoenzymes. Q Rev Biophys. 1988 Aug;21(3):299–329. doi: 10.1017/s0033583500004479. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Vincent S. P., Lowe D. J., Clegg R. A., Garland P. B. Electron-paramagnetic-resonance studies on the molybdenum of nitrate reductase from Escherichia coli K12. Biochem J. 1976 Apr 1;155(1):201–203. doi: 10.1042/bj1550201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. H. Properties of Bromphenol Blue as an Electron Donor for Higher Plant NADH: Nitrate Reductase. Plant Physiol. 1986 Nov;82(3):729–732. doi: 10.1104/pp.82.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. A., Ferguson L. P., Ingraham J. L. Properties of dissimilatory nitrate reductase purified from the denitrifier Pseudomonas aeruginosa. J Bacteriol. 1982 Jul;151(1):162–171. doi: 10.1128/jb.151.1.162-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., MacGregor C. H. Cytochrome b from Escherichia coli nitrate reductase. Its properties and association with the enzyme complex. J Biol Chem. 1983 May 10;258(9):5819–5827. [PubMed] [Google Scholar]
- Chaudhry G. R., MacGregor C. H. Escherichia coli nitrate reductase subunit A: its role as the catalytic site and evidence for its modification. J Bacteriol. 1983 Apr;154(1):387–394. doi: 10.1128/jb.154.1.387-394.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske A., Ferguson S. J. The respiratory nitrate reductase from Paracoccus denitrificans. Molecular characterisation and kinetic properties. Eur J Biochem. 1986 Jul 15;158(2):429–436. doi: 10.1111/j.1432-1033.1986.tb09771.x. [DOI] [PubMed] [Google Scholar]
- Demoss J. A., Fan T. Y., Scott R. H. Characterization of subunit structural alterations which occur during purification of nitrate reductase from Escherichia coli. Arch Biochem Biophys. 1981 Jan;206(1):54–64. doi: 10.1016/0003-9861(81)90065-5. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Robson R. L., Richardson T. H., Miller R. W., Hawkins M. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the VFe protein. Biochem J. 1987 May 15;244(1):197–207. doi: 10.1042/bj2440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George G. N., Turner N. A., Bray R. C., Morpeth F. F., Boxer D. H., Cramer S. P. X-ray-absorption and electron-paramagnetic-resonance spectroscopic studies of the environment of molybdenum in high-pH and low-pH forms of Escherichia coli nitrate reductase. Biochem J. 1989 May 1;259(3):693–700. doi: 10.1042/bj2590693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey C., Gadsby P. M., Thomson A. J., Greenwood C., Coddington A. Electron-paramagnetic-resonance and magnetic-circular-dichroism studies on the formate dehydrogenase-nitrate reductase particle from Pseudomonas aeruginosa. Biochem J. 1987 Apr 1;243(1):241–248. doi: 10.1042/bj2430241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero M. G., Vega J. M., Leadbetter E., Losada M. Preparation and characterization of a soluble nitrate reductase from Azotobacter chroococcum. Arch Mikrobiol. 1973 Jun 25;91(4):287–304. doi: 10.1007/BF00425049. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Bray R. C., Notton B. A., Fido R. J., Hewitt E. J. Studies by electron-paramagnetic-resonance spectroscopy of the molybdenum centre of spinach (Spinacia oleracea) nitrate reductase. Biochem J. 1983 Jul 1;213(1):137–142. doi: 10.1042/bj2130137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., Bray R. C. Quantitative transfer of the molybdenum cofactor from xanthine oxidase and from sulphite oxidase to the deficient enzyme of the nit-1 mutant of Neurospora crassa to yield active nitrate reductase. Biochem J. 1984 Apr 15;219(2):481–493. doi: 10.1042/bj2190481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hoarau J., Hirel B., Nato A. New artificial electron donors for in vitro assay of nitrate reductase isolated from cultured tobacco cells and other organisms. Plant Physiol. 1986 Apr;80(4):946–949. doi: 10.1104/pp.80.4.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard W. D., Solomonson L. P. Quaternary structure of assimilatory NADH:nitrate reductase from Chlorella. J Biol Chem. 1982 Sep 10;257(17):10243–10250. [PubMed] [Google Scholar]
- Hucklesby D. P., Hageman R. H. A staining method for nitrite reductase on polyacrylamide gels after electrophoresis. Anal Biochem. 1973 Dec;56(2):591–592. doi: 10.1016/0003-2697(73)90226-1. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V., Arison B. H. The pterin component of the molybdenum cofactor. Structural characterization of two fluorescent derivatives. J Biol Chem. 1984 May 10;259(9):5414–5422. [PubMed] [Google Scholar]
- Kay C. J., Barber M. J. EPR and kinetic analysis of the interaction of halides and phosphate with nitrate reductase. Biochemistry. 1989 Jul 11;28(14):5750–5758. doi: 10.1021/bi00440a008. [DOI] [PubMed] [Google Scholar]
- Kay C. J., Barber M. J., Notton B. A., Solomonson L. P. Oxidation--reduction midpoint potentials of the flavin, haem and Mo-pterin centres in spinach (Spinacia oleracea L.) nitrate reductase. Biochem J. 1989 Oct 1;263(1):285–287. doi: 10.1042/bj2630285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay C. J., Barber M. J., Solomonson L. P., Kau D., Cannons A. C., Hipkin C. R. Spectroscopic, thermodynamic and kinetic properties of Candida nitratophila nitrate reductase. Biochem J. 1990 Dec 1;272(2):545–548. doi: 10.1042/bj2720545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger B., Meyer O. Structural elements of bactopterin from Pseudomonas carboxydoflava carbon monoxide dehydrogenase. Biochim Biophys Acta. 1987 Apr 30;912(3):357–364. doi: 10.1016/0167-4838(87)90040-9. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Ogura N., Nakagawa H. Limited proteolysis of the nitrate reductase from spinach leaves. J Biol Chem. 1988 Dec 25;263(36):19684–19689. [PubMed] [Google Scholar]
- LOWE R. H., EVANS H. J. PREPARATION AND SOME PROPERTIES OF A SOLUBLE NITRATE REDUCTASE FROM RHIZOBIUM JAPONICUM. Biochim Biophys Acta. 1964 Jun 1;85:377–389. doi: 10.1016/0926-6569(64)90301-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacGregor C. H. Isolation and characterization of nitrate reductase from Escherichia coli. Methods Enzymol. 1978;53:347–355. doi: 10.1016/s0076-6879(78)53040-1. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malofeeva I. V., Kondratieva E. N., Rubin A. B. Ferredoxin-linked nitrate reductase from the phototrophic bacterium Ectothiorhodospira shaposhnikovii. FEBS Lett. 1975 May 1;53(2):188–189. doi: 10.1016/0014-5793(75)80016-0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan K. V., Johnson J. L. The pterin molybdenum cofactors. J Biol Chem. 1992 May 25;267(15):10199–10202. [PubMed] [Google Scholar]
- Rajagopalan K. V. Novel aspects of the biochemistry of the molybdenum cofactor. Adv Enzymol Relat Areas Mol Biol. 1991;64:215–290. doi: 10.1002/9780470123102.ch5. [DOI] [PubMed] [Google Scholar]
- Seki S., Hattori Y., Hasegawa T., Haraguchi H., Ishimoto M. Studies on nitrate reductase of Clostridium perfringens. IV. Identification of metals, molybdenum cofactor, and iron-sulfur cluster. J Biochem. 1987 Feb;101(2):503–509. doi: 10.1093/oxfordjournals.jbchem.a121937. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Barber M. J., Robbins A. P., Oaks A. Functional domains of assimilatory NADH:nitrate reductase from Chlorella. J Biol Chem. 1986 Aug 25;261(24):11290–11294. [PubMed] [Google Scholar]
- Solomonson L. P., McCreery M. J. Radiation inactivation of assimilatory NADH:nitrate reductase from Chlorella. Catalytic and physical sizes of functional units. J Biol Chem. 1986 Jan 15;261(2):806–810. [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Turner N., Ballard A. L., Bray R. C., Ferguson S. Investigation by electron paramagnetic resonance spectroscopy of the molybdenum centre of respiratory nitrate reductase from Paracoccus denitrificans. Biochem J. 1988 Jun 15;252(3):925–926. doi: 10.1042/bj2520925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventom A. M., Deistung J., Bray R. C. The isolation of demolybdo xanthine oxidase from bovine milk. Biochem J. 1988 Nov 1;255(3):949–956. doi: 10.1042/bj2550949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila R., Bárcena J. A., Llobell A., Paneque A. Characterization of a membrane-bound nitrate reductase from Azotobacter chroococcum. Biochem Biophys Res Commun. 1977 Apr 11;75(3):682–688. doi: 10.1016/0006-291x(77)91526-1. [DOI] [PubMed] [Google Scholar]
- Vincent S. P., Bray R. C. Electron-paramagnetic-resonance studies on nitrate reductase from Escherichia coli K12. Biochem J. 1978 Jun 1;171(3):639–647. doi: 10.1042/bj1710639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- van Riet J., van Ed J. H., Wever R., van Gelder B. F., Planta R. J. Characterization of the respiratory nitrate reductase of Klebsiella aerogenes as a molybdenum-containing iron-sulfur enzyme. Biochim Biophys Acta. 1975 Oct 20;405(2):306–317. doi: 10.1016/0005-2795(75)90096-3. [DOI] [PubMed] [Google Scholar]