Abstract
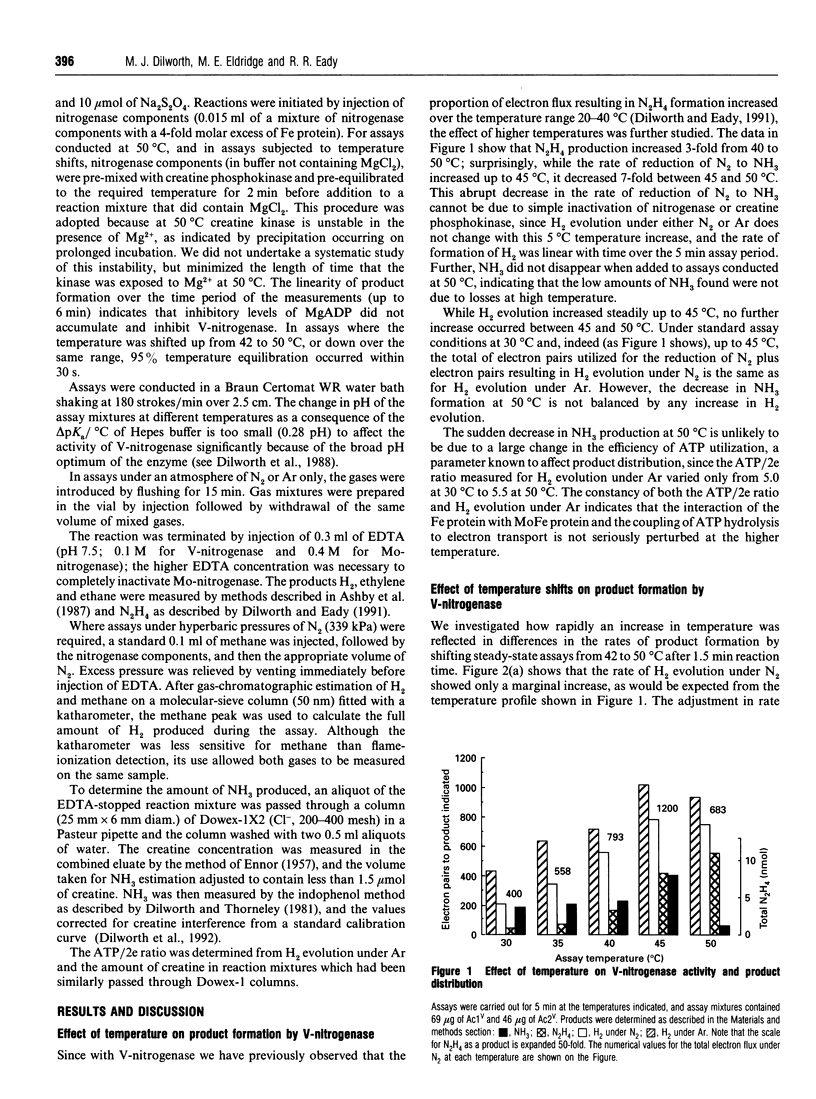
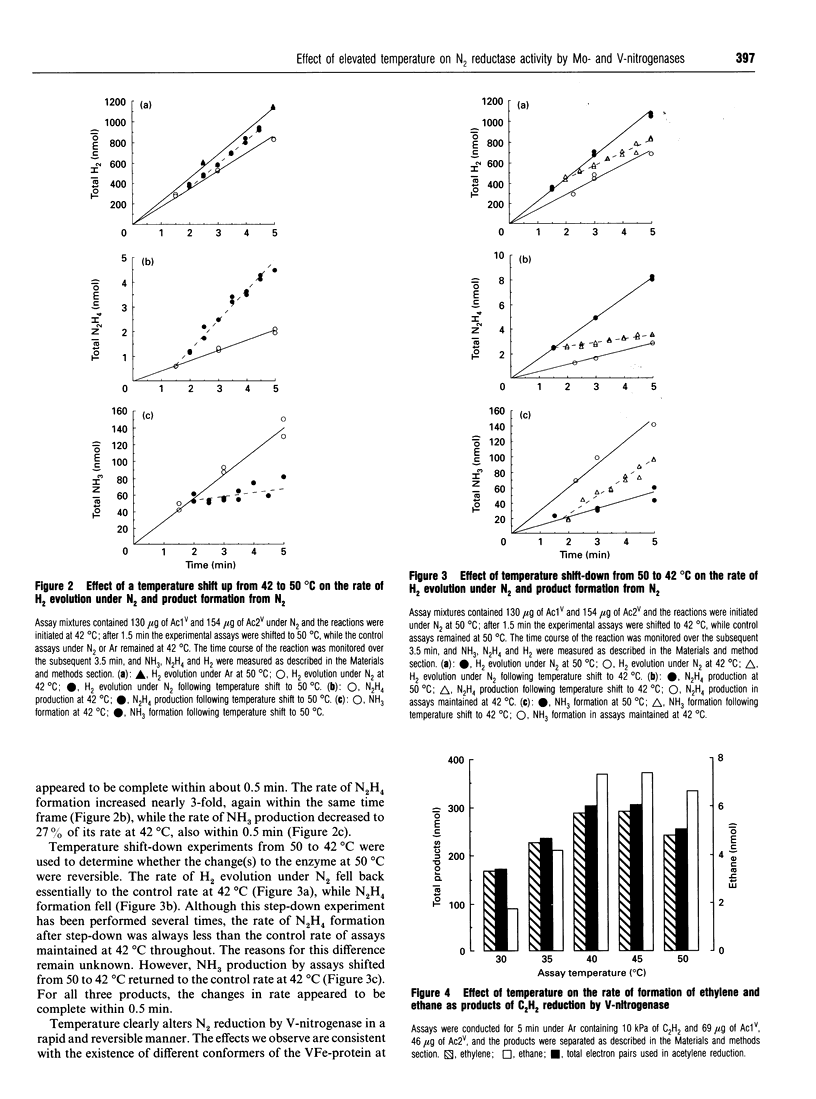
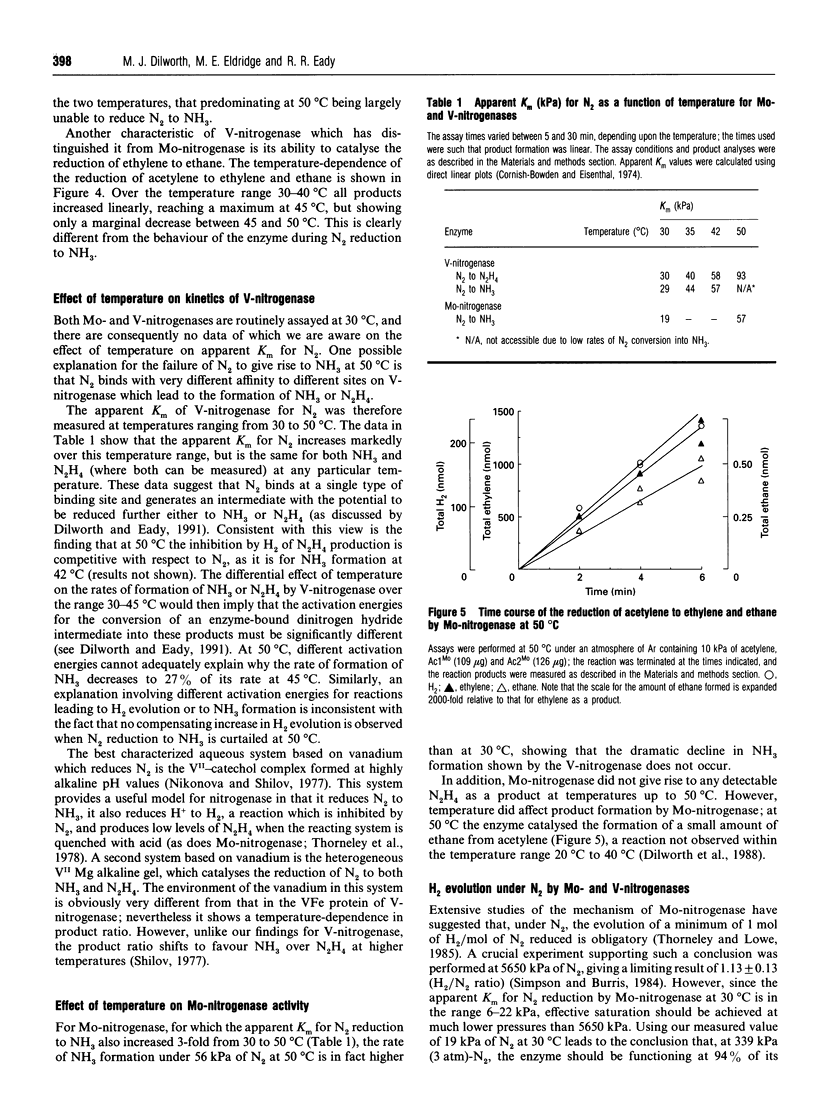
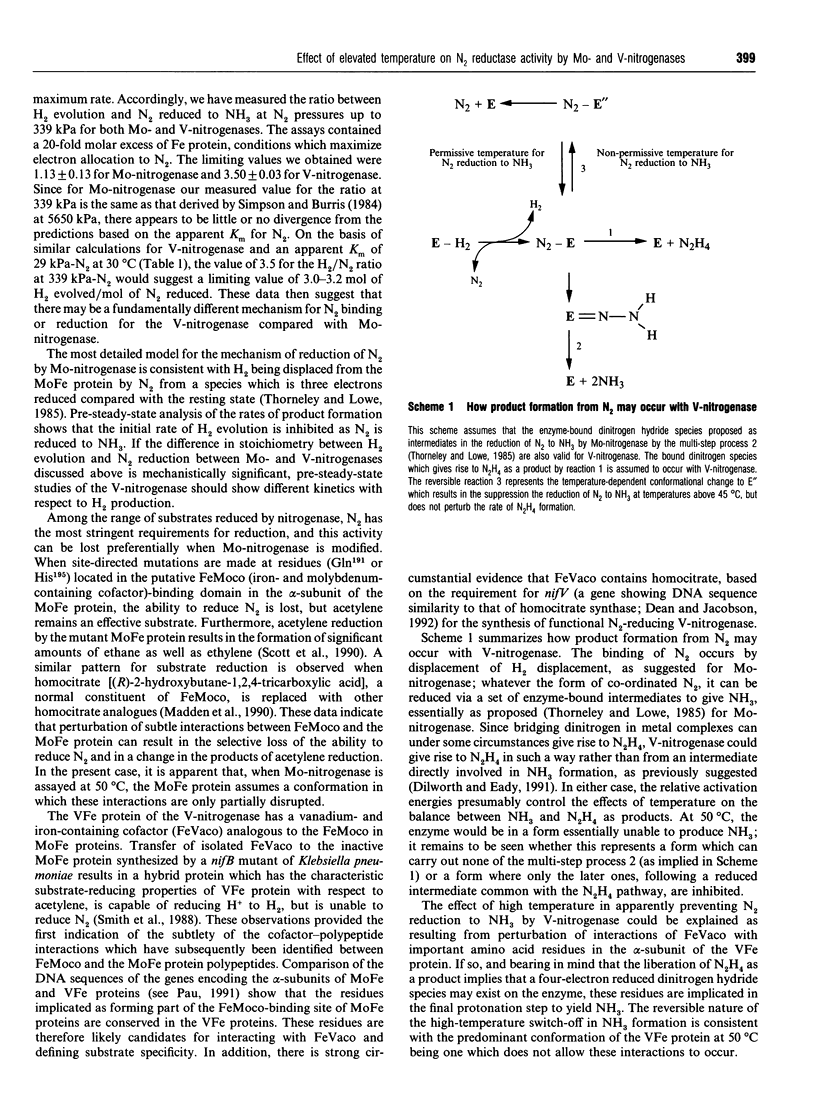
During the reduction of N2 by V-nitrogenase at 30 degrees C, some hydrazine (N2H4) is formed as a product in addition to NH3 [Dilworth and Eady (1991) Biochem. J. 277, 465-468]. We show here the following. (1) That over the temperature range 30-45 degrees C the apparent Km for the reduction of N2 to yield these products is the same, but increases from 30 to 58 kPa of N2. On increasing the temperature from 45 degrees C to 50 degrees C, little change occurred in the rate of reduction of protons to H2; the rate of N2H4 production increased, but the rate of NH3 formation decreased 7-fold. (2) Temperature-shift experiments from 42 to 50 degrees C or from 50 to 42 degrees C showed that this selective loss of the ability to reduce N2 to NH3 was reversible. The effects we observe are consistent with the existence of different conformers of the VFe-protein at the two temperatures, that predominating at 50 degrees C being largely unable to reduce N2 to ammonia. (3) Measurement of the ratio between H2 evolution and N2 reduced to NH3 at N2 pressures up to 339 kPa for both Mo- and V-nitrogenases gave limiting H2/N2 values of 1.13 +/- 0.13 for Mo-nitrogenase and 3.50 +/- 0.03 for V-nitrogenase. Since for Mo-nitrogenase our measured value for the ratio at 339 kPa is the same as that derived by Simpson and Burris [(1984) Science 224, 1095-1097] at 5650 kPa, there appears to be little or no divergence from the predictions based on the apparent Km for N2. These data then suggest that there may be a fundamentally different mechanism for N2 binding to V-nitrogenase compared with Mo-nitrogenase. (4) We did not detect any N2H4 as a product of N2 reduction by Mo-nitrogenase over the temperature range investigated; however, at 50 degrees C this system reduced acetylene (C2H2) to yield some ethane (C2H6), in addition to ethylene (C2H4), a reaction normally associated with Mo-independent nitrogenases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby G. A., Dilworth M. J., Thorneley R. N. Klebsiella pneumoniae nitrogenase. Inhibition of hydrogen evolution by ethylene and the reduction of ethylene to ethane. Biochem J. 1987 Nov 1;247(3):547–554. doi: 10.1042/bj2470547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. C. The nitrogenase system from Azotobacter: activation energy and divalent cation requirement. Biochim Biophys Acta. 1969 Feb 11;171(2):253–259. doi: 10.1016/0005-2744(69)90158-2. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J., Eady R. R., Eldridge M. E. The vanadium nitrogenase of Azotobacter chroococcum. Reduction of acetylene and ethylene to ethane. Biochem J. 1988 Feb 1;249(3):745–751. doi: 10.1042/bj2490745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J., Eady R. R. Hydrazine is a product of dinitrogen reduction by the vanadium-nitrogenase from Azotobacter chroococcum. Biochem J. 1991 Jul 15;277(Pt 2):465–468. doi: 10.1042/bj2770465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J., Thorneley R. N. Nitrogenase of Klebsiella pneumoniae. Hydrazine is a product of azide reduction. Biochem J. 1981 Mar 1;193(3):971–983. doi: 10.1042/bj1930971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Richardson T. H., Miller R. W., Hawkins M., Lowe D. J. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the Fe protein. Biochem J. 1988 Nov 15;256(1):189–196. doi: 10.1042/bj2560189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Robson R. L., Richardson T. H., Miller R. W., Hawkins M. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the VFe protein. Biochem J. 1987 May 15;244(1):197–207. doi: 10.1042/bj2440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden M. S., Kindon N. D., Ludden P. W., Shah V. K. Diastereomer-dependent substrate reduction properties of a dinitrogenase containing 1-fluorohomocitrate in the iron-molybdenum cofactor. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6517–6521. doi: 10.1073/pnas.87.17.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. W., Eady R. R. Molybdenum and vanadium nitrogenases of Azotobacter chroococcum. Low temperature favours N2 reduction by vanadium nitrogenase. Biochem J. 1988 Dec 1;256(2):429–432. doi: 10.1042/bj2560429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. J., May H. D., Newton W. E., Brigle K. E., Dean D. R. Role for the nitrogenase MoFe protein alpha-subunit in FeMo-cofactor binding and catalysis. Nature. 1990 Jan 11;343(6254):188–190. doi: 10.1038/343188a0. [DOI] [PubMed] [Google Scholar]
- Simpson F. B., Burris R. H. A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science. 1984 Jun 8;224(4653):1095–1097. doi: 10.1126/science.6585956. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Eady R. R., Lowe D. J., Gormal C. The vanadium-iron protein of vanadium nitrogenase from Azotobacter chroococcum contains an iron-vanadium cofactor. Biochem J. 1988 Feb 15;250(1):299–302. doi: 10.1042/bj2500299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Eady R. R., Yates M. G. Nitrogenases of Klebsiella pneumoniae and Azotobacter chroococum. Complex formation between the component proteins. Biochim Biophys Acta. 1975 Oct 22;403(2):269–284. doi: 10.1016/0005-2744(75)90057-1. [DOI] [PubMed] [Google Scholar]
- Watt G. D., Burns A. Kinetics of dithionite ion utilization and ATP hydrolysis for reactions catalyzed by the nitrogenase complex from Azotobacter vinelandii. Biochemistry. 1977 Jan 25;16(2):264–270. doi: 10.1021/bi00621a017. [DOI] [PubMed] [Google Scholar]
- Yates M. G., Planqué K. Nitrogenase from Azotobacter chroococcum. Purification and properties of the component proteins. Eur J Biochem. 1975 Dec 15;60(2):467–476. doi: 10.1111/j.1432-1033.1975.tb21025.x. [DOI] [PubMed] [Google Scholar]


