Abstract
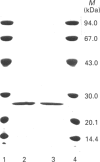
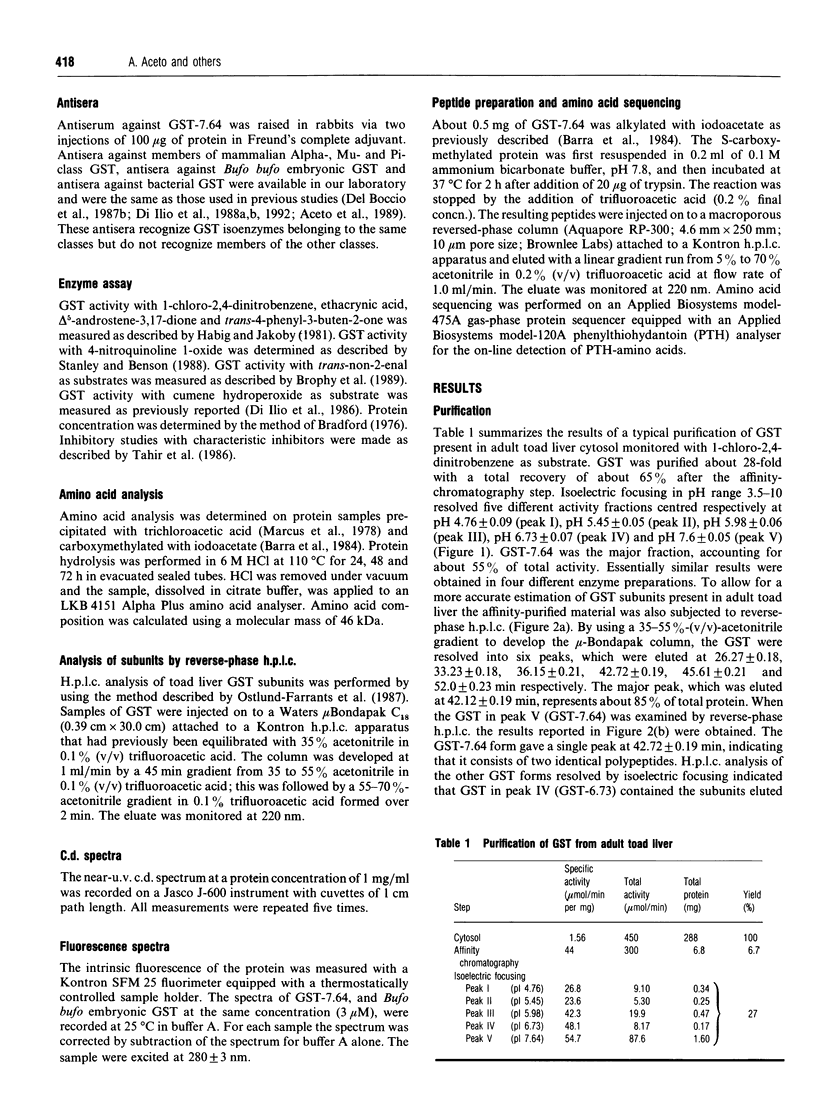
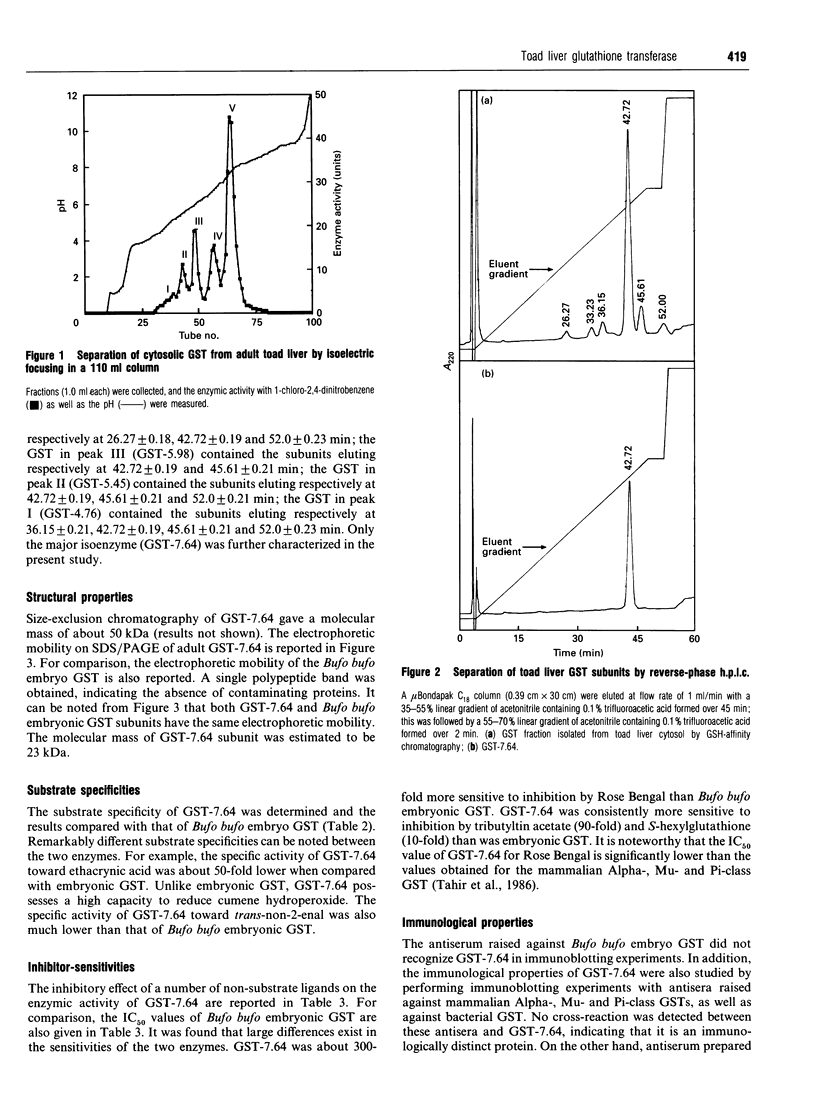
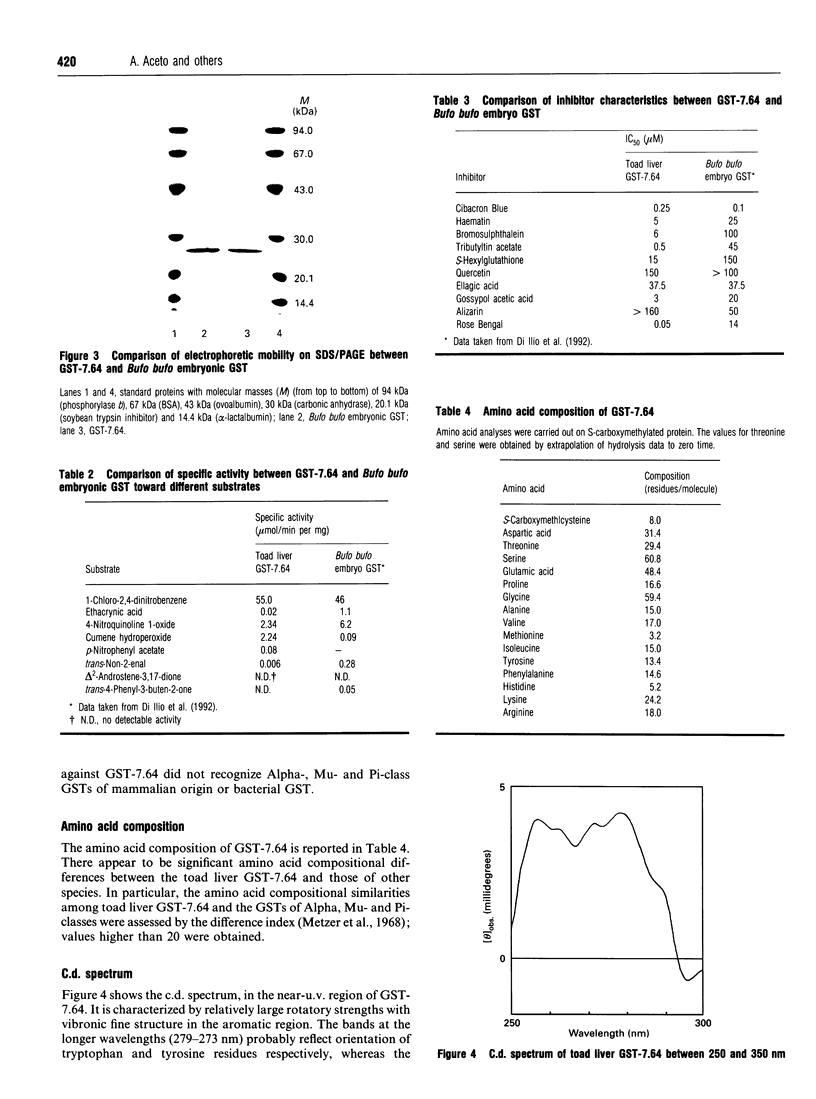
Five forms of glutathione transferase (GST) were resolved from the cytosol of adult common toad (Bufo bufo) liver by GSH-affinity chromatography followed by isoelectric focusing. The major enzyme (GST-7.64; 55% of total activity bound to the column) has a pI value of 7.64, is composed of two subunits each with a molecular mass of 23 kDa, and has the N-terminal amino acid residue blocked. GST-7.64 has also been characterized with respect to amino acid composition, substrate specificity, inhibition characteristics, c.d. spectra and immunological reactivity. The N-terminal sequence of some peptides obtained after tryptic digestion has also been determined. All together the results obtained suggest that the major toad liver GST is distinct from any known GST, including microbial, plant and mammalian GSTs.
Full text
PDF
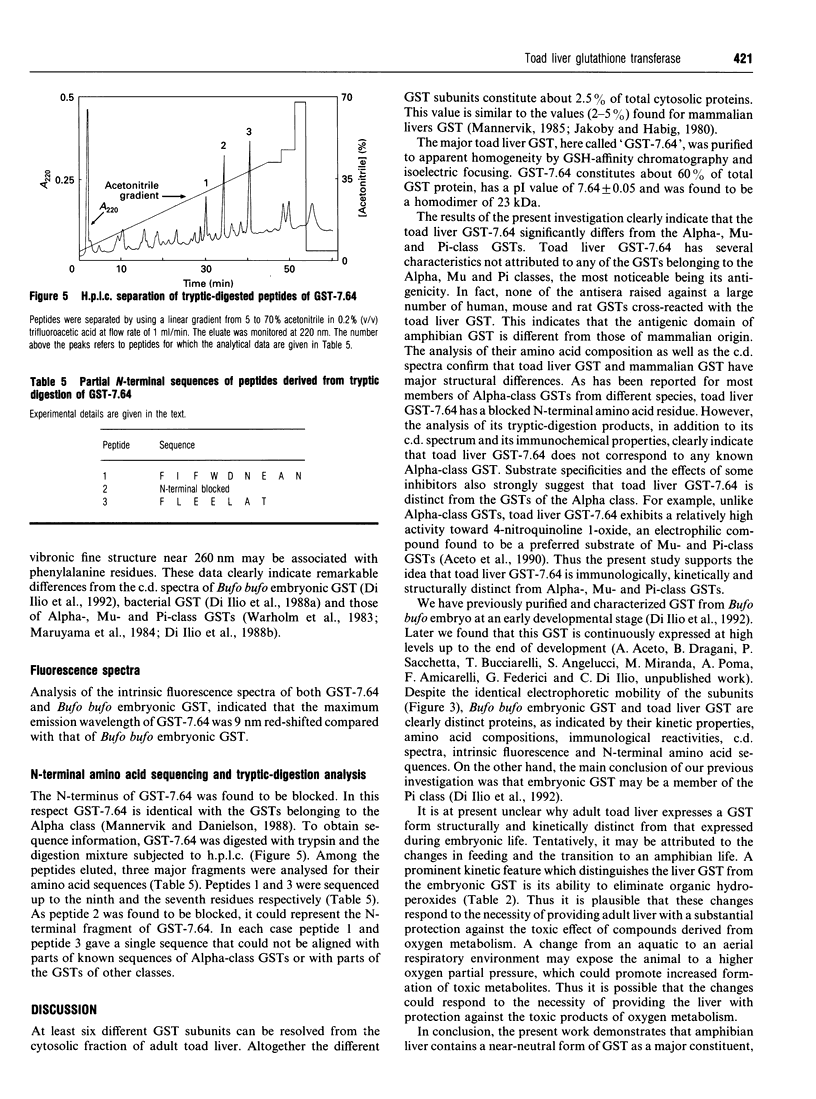

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceto A., Di Ilio C., Angelucci S., Tenaglia R., Zezza A., Caccuri A. M., Federici G. Glutathione-related enzyme activities in testis of patients with malignant diseases. Clin Chim Acta. 1989 Jul 31;183(1):83–86. doi: 10.1016/0009-8981(89)90276-3. [DOI] [PubMed] [Google Scholar]
- Aceto A., Di Ilio C., Lo Bello M., Bucciarelli T., Angelucci S., Federici G. Differential activity of human, rat, mouse and bacteria glutathione transferase isoenzymes towards 4-nitroquinoline 1-oxide. Carcinogenesis. 1990 Dec;11(12):2267–2269. doi: 10.1093/carcin/11.12.2267. [DOI] [PubMed] [Google Scholar]
- Barra D., Schinina M. E., Simmaco M., Bannister J. V., Bannister W. H., Rotilio G., Bossa F. The primary structure of human liver manganese superoxide dismutase. J Biol Chem. 1984 Oct 25;259(20):12595–12601. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brophy P. M., Southan C., Barrett J. Glutathione transferases in the tapeworm Moniezia expansa. Biochem J. 1989 Sep 15;262(3):939–946. doi: 10.1042/bj2620939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boccio G., Di Ilio C., Alin P., Jörnvall H., Mannervik B. Identification of a novel glutathione transferase in human skin homologous with class alpha glutathione transferase 2-2 in the rat. Biochem J. 1987 May 15;244(1):21–25. doi: 10.1042/bj2440021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ilio C., Aceto A., Bucciarelli T., Dragani B., Angelucci S., Miranda M., Poma A., Amicarelli F., Barra D., Federici G. Glutathione transferase isoenzymes from Bufo bufo embryos at an early developmental stage. Biochem J. 1992 Apr 1;283(Pt 1):217–222. [PMC free article] [PubMed] [Google Scholar]
- Di Ilio C., Aceto A., Del Boccio G., Casalone E., Pennelli A., Federici G. Purification and characterization of five forms of glutathione transferase from human uterus. Eur J Biochem. 1988 Feb 1;171(3):491–496. doi: 10.1111/j.1432-1033.1988.tb13816.x. [DOI] [PubMed] [Google Scholar]
- Di Ilio C., Aceto A., Piccolomini R., Allocati N., Faraone A., Bucciarelli T., Barra D., Federici G. Purification and characterization of a novel glutathione transferase from Serratia marcescens. Biochim Biophys Acta. 1991 Apr 8;1077(2):141–146. doi: 10.1016/0167-4838(91)90050-a. [DOI] [PubMed] [Google Scholar]
- Di Ilio C., Aceto A., Piccolomini R., Allocati N., Faraone A., Cellini L., Ravagnan G., Federici G. Purification and characterization of three forms of glutathione transferase from Proteus mirabilis. Biochem J. 1988 Nov 1;255(3):971–975. doi: 10.1042/bj2550971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ilio C., Sacchetta P., Lo Bello M., Caccuri A. M., Federici G. Selenium independent glutathione peroxidase activity associated with cationic forms of glutathione transferase in human heart. J Mol Cell Cardiol. 1986 Sep;18(9):983–991. doi: 10.1016/s0022-2828(86)80012-8. [DOI] [PubMed] [Google Scholar]
- Dominey R. J., Nimmo I. A., Cronshaw A. D., Hayes J. D. The major glutathione S-transferase in salmonid fish livers is homologous to the mammalian pi-class GST. Comp Biochem Physiol B. 1991;100(1):93–98. doi: 10.1016/0305-0491(91)90090-z. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hiratsuka A., Sebata N., Kawashima K., Okuda H., Ogura K., Watabe T., Satoh K., Hatayama I., Tsuchida S., Ishikawa T. A new class of rat glutathione S-transferase Yrs-Yrs inactivating reactive sulfate esters as metabolites of carcinogenic arylmethanols. J Biol Chem. 1990 Jul 15;265(20):11973–11981. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Marcus C. J., Habig W. H., Jakoby W. B. Glutathione transferase from human erythrocytes. Nonidentity with the enzymes from liver. Arch Biochem Biophys. 1978 Jun;188(2):287–293. doi: 10.1016/s0003-9861(78)80011-3. [DOI] [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Gilmore K. S., Coles B., Dalton K., Hulbert P. B., Ketterer B. Structural distinction of rat GSH transferase subunit 10. Biochem J. 1991 Mar 1;274(Pt 2):619–619. doi: 10.1042/bj2740619a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer T. J., Tiemeier D. C., Jaworski E. G. Purification and characterization of corn glutathione S-transferase. Biochemistry. 1983 Mar 1;22(5):1068–1072. doi: 10.1021/bi00274a011. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Stanley J. S., Benson A. M. The conjugation of 4-nitroquinoline 1-oxide, a potent carcinogen, by mammalian glutathione transferases. 4-Nitroquinoline 1-oxide conjugation by human, rat and mouse liver cytosols, extrahepatic organs of mice and purified mouse glutathione transferase isoenzymes. Biochem J. 1988 Nov 15;256(1):303–306. doi: 10.1042/bj2560303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir M. K., Guthenberg C., Mannervik B. Inhibitors for distinction of three types of human glutathione transferase. FEBS Lett. 1985 Feb 25;181(2):249–252. doi: 10.1016/0014-5793(85)80269-6. [DOI] [PubMed] [Google Scholar]
- Toung Y. P., Hsieh T. S., Tu C. P. Drosophila glutathione S-transferase 1-1 shares a region of sequence homology with the maize glutathione S-transferase III. Proc Natl Acad Sci U S A. 1990 Jan;87(1):31–35. doi: 10.1073/pnas.87.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]